Not A love story Head and neck cancer







![Tobacco Use in the US, 1900 -1999 100 5000 Per Capita Cigarette Consumption[1] Per Tobacco Use in the US, 1900 -1999 100 5000 Per Capita Cigarette Consumption[1] Per](https://slidetodoc.com/presentation_image_h2/cfb61222c4d5dba96c6073147f5a65a9/image-11.jpg)






![[TITLE] [TITLE]](https://slidetodoc.com/presentation_image_h2/cfb61222c4d5dba96c6073147f5a65a9/image-58.jpg)
![[TITLE] [TITLE]](https://slidetodoc.com/presentation_image_h2/cfb61222c4d5dba96c6073147f5a65a9/image-59.jpg)
![[TITLE] [TITLE]](https://slidetodoc.com/presentation_image_h2/cfb61222c4d5dba96c6073147f5a65a9/image-60.jpg)



- Slides: 69

Not A love story Head and neck cancer Dr Marco Matos Gold Coast Cancer Care, Gold Coast University Hospital and Pacific Private Group

A love story?

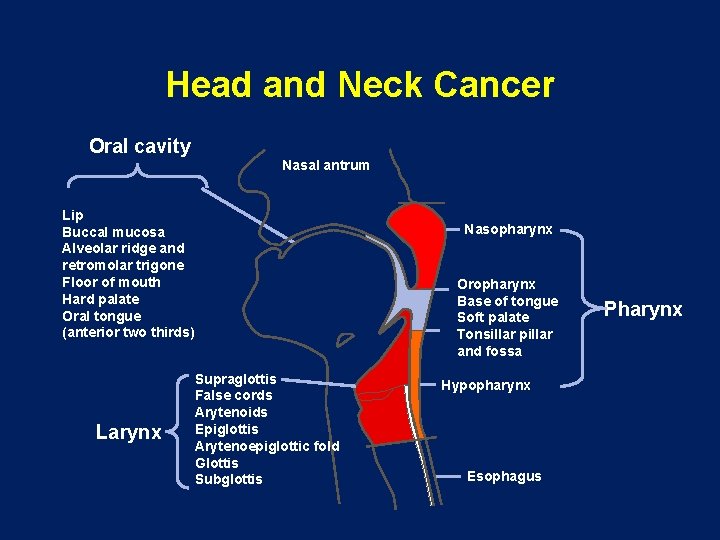
Head and Neck Cancer Oral cavity Nasal antrum Lip Buccal mucosa Alveolar ridge and retromolar trigone Floor of mouth Hard palate Oral tongue (anterior two thirds) Larynx Nasopharynx Oropharynx Base of tongue Soft palate Tonsillar pillar and fossa Supraglottis False cords Arytenoids Epiglottis Arytenoepiglottic fold Glottis Subglottis Hypopharynx Esophagus Pharynx

Head and Neck Cancer Estimated 39, 250 cases in US in 2005* Ø 5 th leading cancer in US Ø 10% of all cancers worldwide 11, 000 deaths— 3% of all cancer deaths Patients typically aged > 50 yrs, with tobacco and alcohol use Emerging cohort of HPV-positive cancers in the OP (oropharynx) Histology: > 90% squamous cell carcinoma Early-stage disease (I, II) curable: > 80% Locally advanced disease has poorer prognosis Ø 5 -yr survival rate: < 40% *Oral cavity, pharynx, and larynx.

INCIDENCE AND MORTALITY IN AUSTRALIA - 2006 2, 756 new cases (2. 6 per cent of all new cancer cases). Most common in males= 2, 059 than females = 697. The lifetime risk of developing a head or neck cancer before the age of 75 is 1 in 95 The most common forms of head and neck cancers in 2003* were: o Larynx (557 cases; 498 male, 59 female) o Tongue (492 cases; 343 male, 149 female) o Mouth (466 cases; 287 male, 179 female). In 2007, head and neck cancers were responsible for 898 deaths in Australia, accounting for 2. 3 per cent of all cancer deaths


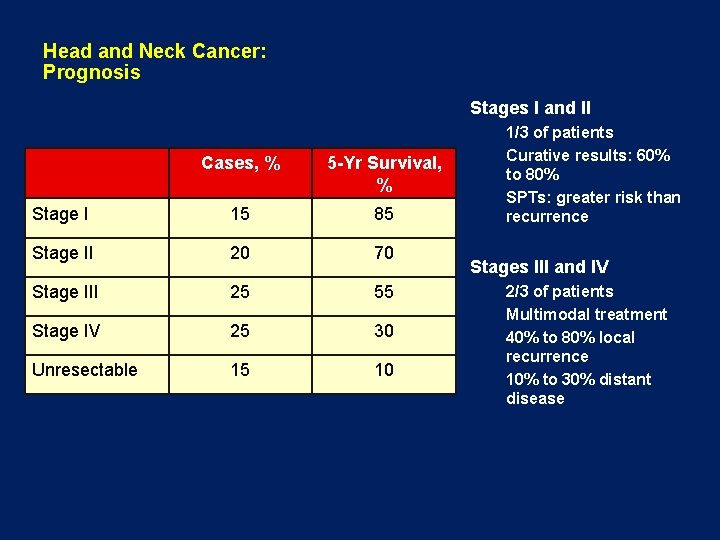
Head and Neck Cancer: Prognosis Stages I and II Cases, % 5 -Yr Survival, % Stage I 15 85 Stage II 20 70 Stage III 25 55 Stage IV 25 30 Unresectable 15 10 1/3 of patients Curative results: 60% to 80% SPTs: greater risk than recurrence Stages III and IV 2/3 of patients Multimodal treatment 40% to 80% local recurrence 10% to 30% distant disease


Risk Factors Age (majority of patients: older than 50 yrs) Tobacco Alcohol Viral (25% to 35% of patients with SCC) Ø EBV causes endemic Nasopharyngeal cancer (internationally greater representation)[1] Ø HPV (increasing incidence in Europe and North America; associated with > 50% of oropharyngeal tumors) 1. National Cancer Institute. Fact sheet on head and neck cancers. 2. Dayyani F, et al. Head Neck Oncol. 2010; 2: 15. 3. Chaturvedi AK, et al. J Clin Oncol. 2011; 29: 4294 -4301.

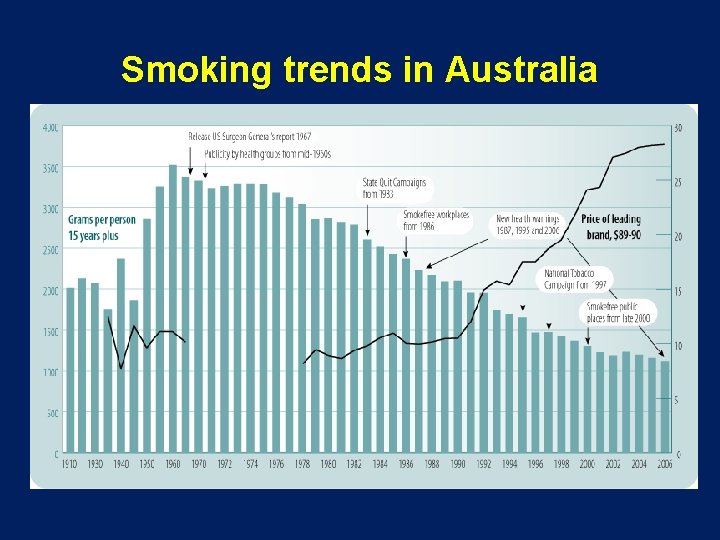
Smoking trends in Australia
![Tobacco Use in the US 1900 1999 100 5000 Per Capita Cigarette Consumption1 Per Tobacco Use in the US, 1900 -1999 100 5000 Per Capita Cigarette Consumption[1] Per](https://slidetodoc.com/presentation_image_h2/cfb61222c4d5dba96c6073147f5a65a9/image-11.jpg)
Tobacco Use in the US, 1900 -1999 100 5000 Per Capita Cigarette Consumption[1] Per capita cigarette consumption 90 4000 80 3500 70 3000 60 Male lung cancer death rate 2200 50 2000 40 1500 30 1000 Female lung cancer death rate 500 10 0 19 05 19 10 19 15 19 20 19 25 19 30 19 35 19 40 19 45 19 50 19 55 19 60 19 65 19 70 19 75 19 80 19 85 19 90 19 95 0 20 Age-Adjusted Lung Cancer Death Rates*[2] 4500 Yrs *Age-adjusted to 2000 US standard population. 1. CDC. MMWR Morb Mortal Wkly Rep. 1999; 48: 986 -993. 2. Death rates: US Mortality Public Use Tapes, 1960 -1999, US Mortality Volumes, 1930 -1959, National Center for Health Statistics, Centers for Disease Control and Prevention, 2001.

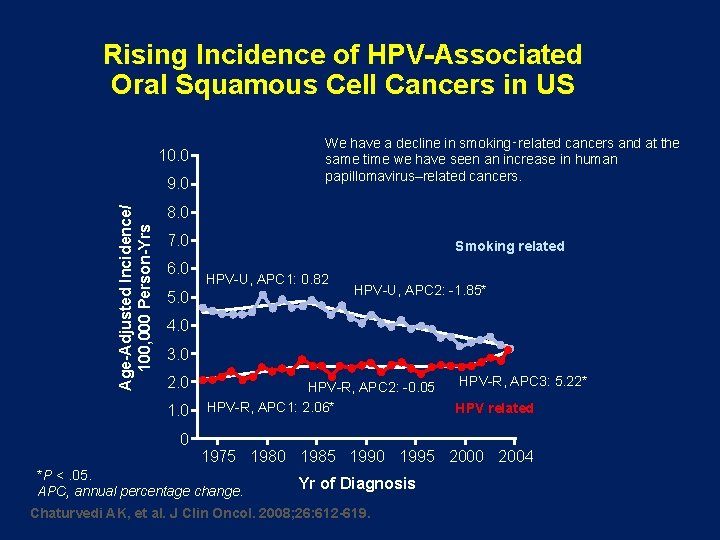
Rising Incidence of HPV-Associated Oral Squamous Cell Cancers in US We have a decline in smoking‑related cancers and at the same time we have seen an increase in human papillomavirus–related cancers. 10. 0 Age-Adjusted Incidence/ 100, 000 Person-Yrs 9. 0 8. 0 7. 0 6. 0 Smoking related HPV-U, APC 1: 0. 82 5. 0 HPV-U, APC 2: -1. 85* 4. 0 3. 0 2. 0 1. 0 0 HPV-R, APC 2: -0. 05 HPV-R, APC 1: 2. 06* HPV-R, APC 3: 5. 22* HPV related 1975 1980 1985 1990 1995 2000 2004 *P <. 05. APC, annual percentage change. Yr of Diagnosis Chaturvedi AK, et al. J Clin Oncol. 2008; 26: 612 -619.

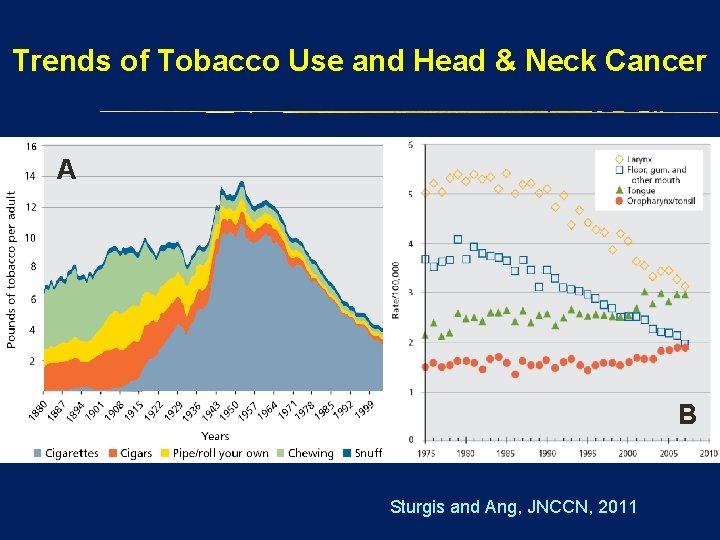
Trends of Tobacco Use and Head & Neck Cancer A B Sturgis and Ang, JNCCN, 2011

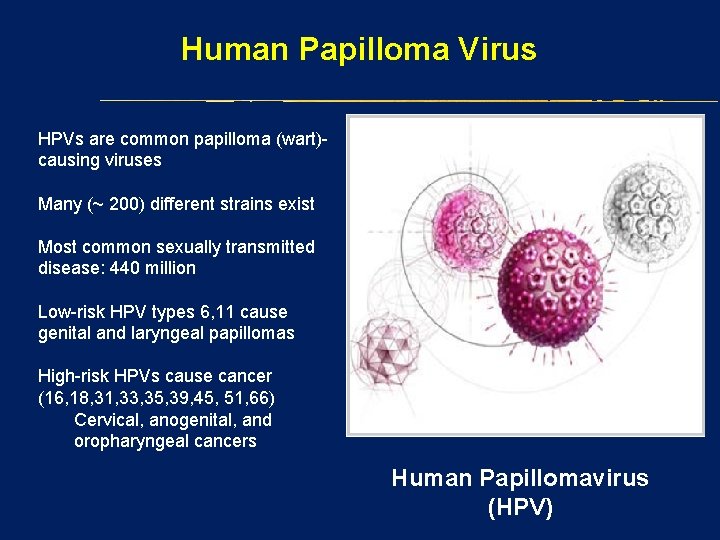
Human Papilloma Virus HPVs are common papilloma (wart)causing viruses Many (~ 200) different strains exist Most common sexually transmitted disease: 440 million Low-risk HPV types 6, 11 cause genital and laryngeal papillomas High-risk HPVs cause cancer (16, 18, 31, 33, 35, 39, 45, 51, 66) Cervical, anogenital, and oropharyngeal cancers Human Papillomavirus (HPV)

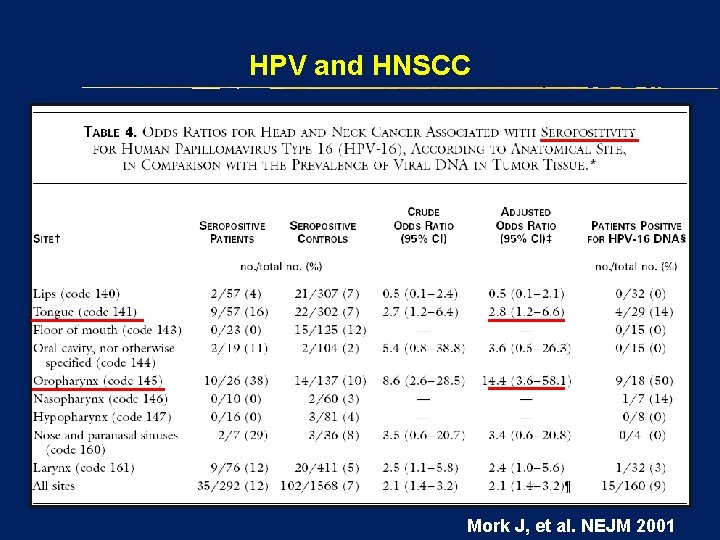
HPV and HNSCC Mork J, et al. NEJM 2001

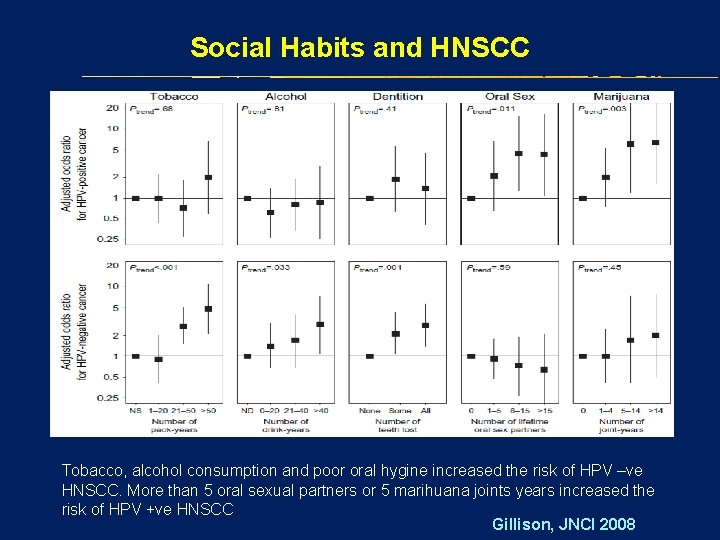
Social Habits and HNSCC Tobacco, alcohol consumption and poor oral hygine increased the risk of HPV –ve HNSCC. More than 5 oral sexual partners or 5 marihuana joints years increased the risk of HPV +ve HNSCC Gillison, JNCI 2008

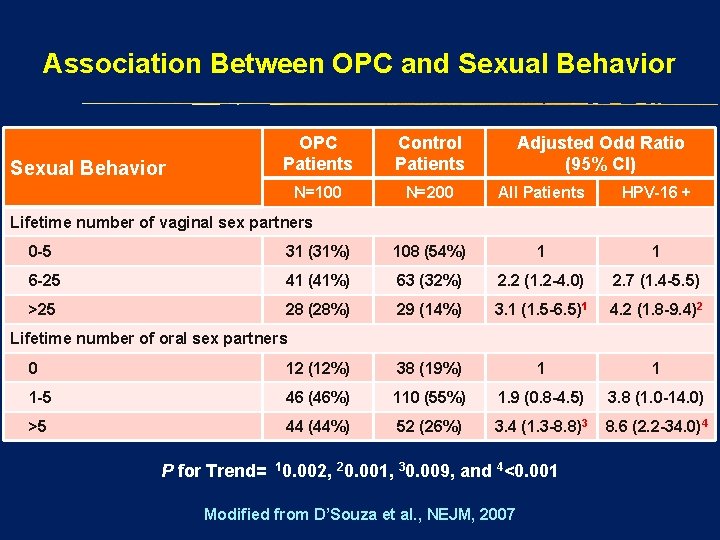
Association Between OPC and Sexual Behavior OPC Patients Control Patients Adjusted Odd Ratio (95% CI) N=100 N=200 All Patients HPV-16 + Lifetime number of vaginal sex partners 0 -5 31 (31%) 108 (54%) 1 1 6 -25 41 (41%) 63 (32%) 2. 2 (1. 2 -4. 0) 2. 7 (1. 4 -5. 5) >25 28 (28%) 29 (14%) 3. 1 (1. 5 -6. 5)1 4. 2 (1. 8 -9. 4)2 Lifetime number of oral sex partners 0 12 (12%) 38 (19%) 1 1 1 -5 46 (46%) 110 (55%) 1. 9 (0. 8 -4. 5) 3. 8 (1. 0 -14. 0) >5 44 (44%) 52 (26%) 3. 4 (1. 3 -8. 8)3 8. 6 (2. 2 -34. 0)4 P for Trend= 10. 002, 20. 001, 30. 009, and 4<0. 001 Modified from D’Souza et al. , NEJM, 2007

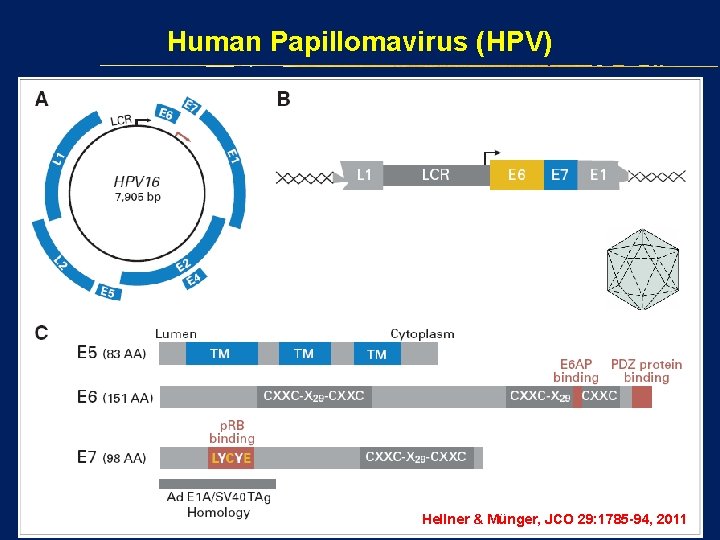
Human Papillomavirus (HPV) Hellner & Münger, JCO 29: 1785 -94, 2011

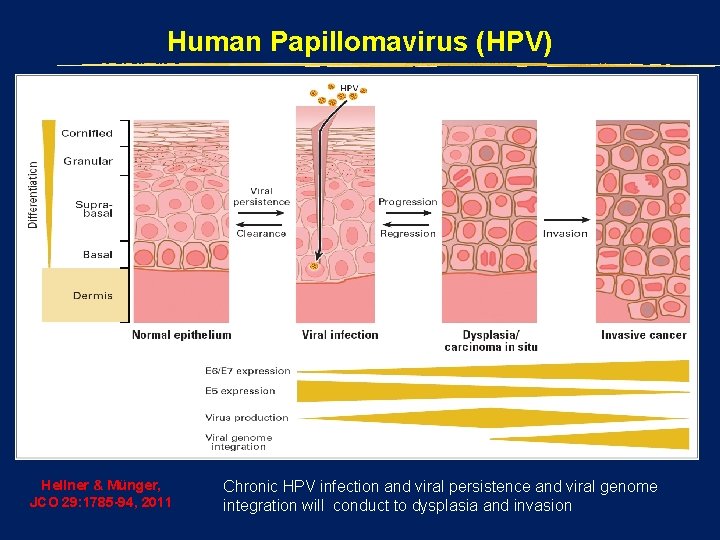
Human Papillomavirus (HPV) Hellner & Münger, JCO 29: 1785 -94, 2011 Chronic HPV infection and viral persistence and viral genome integration will conduct to dysplasia and invasion

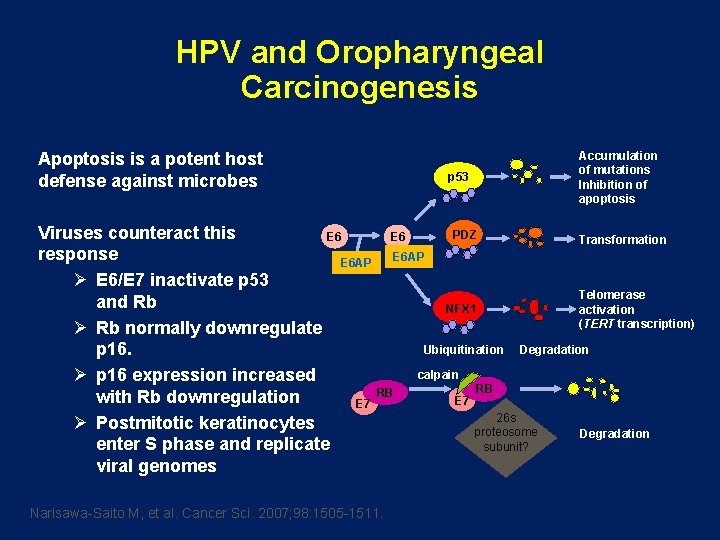
HPV and Oropharyngeal Carcinogenesis Apoptosis is a potent host defense against microbes p 53 Accumulation of mutations Inhibition of apoptosis Viruses counteract this PDZ E 6 Transformation response E 6 AP Ø E 6/E 7 inactivate p 53 Telomerase and Rb NFX 1 activation (TERT transcription) Ø Rb normally downregulate Ubiquitination Degradation p 16. calpain Ø p 16 expression increased RB RB with Rb downregulation E 7 26 s Ø Postmitotic keratinocytes proteosome Degradation enter S phase and replicate subunit? viral genomes Narisawa-Saito M, et al. Cancer Sci. 2007; 98: 1505 -1511.

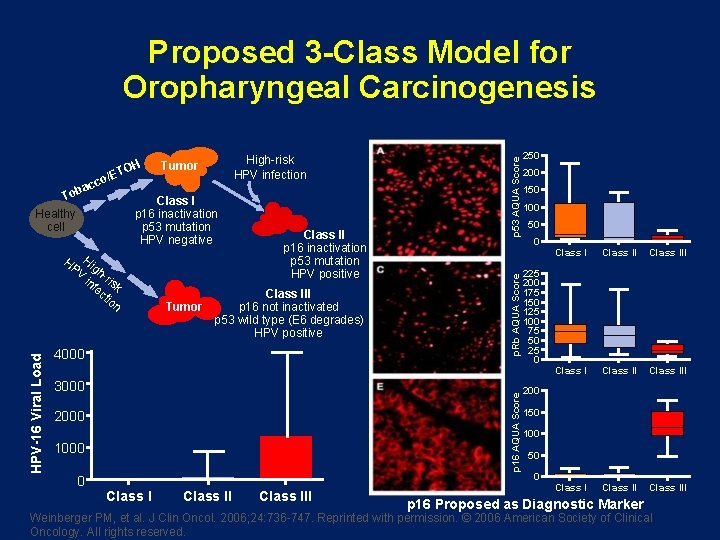
/ET To Healthy cell Class I p 16 inactivation p 53 mutation HPV negative HP Hig V h-r in is fe k ct io n Tumor Class II p 16 inactivation p 53 mutation HPV positive Class III p 16 not inactivated p 53 wild type (E 6 degrades) HPV positive 4000 3000 p 16 AQUA Score HPV-16 Viral Load High-risk HPV infection Tumor 2000 1000 0 p. Rb AQUA Score co bac OH p 53 AQUA Score Proposed 3 -Class Model for Oropharyngeal Carcinogenesis Class III 250 200 150 100 50 0 225 200 175 150 125 100 75 50 25 0 Class II Class III Class III 200 150 100 50 0 p 16 Proposed as Diagnostic Marker Weinberger PM, et al. J Clin Oncol. 2006; 24: 736 -747. Reprinted with permission. © 2006 American Society of Clinical Oncology. All rights reserved.

Clinical Features & Assay Methods

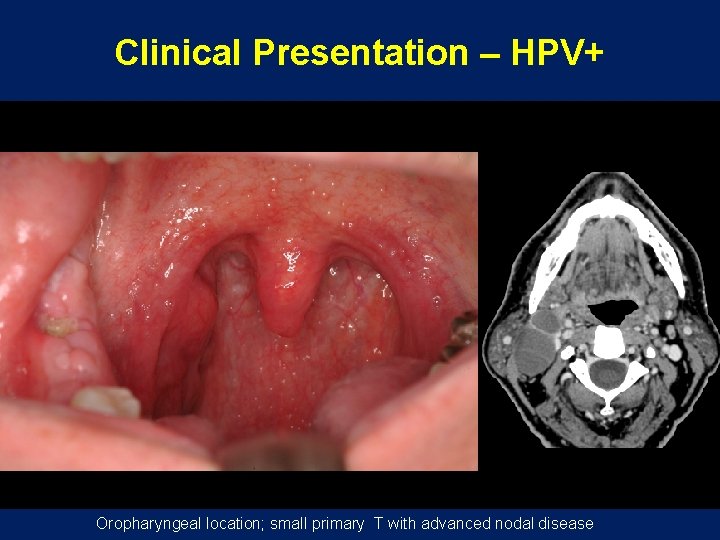
Clinical Presentation – HPV+ Oropharyngeal location; small primary T with advanced nodal disease

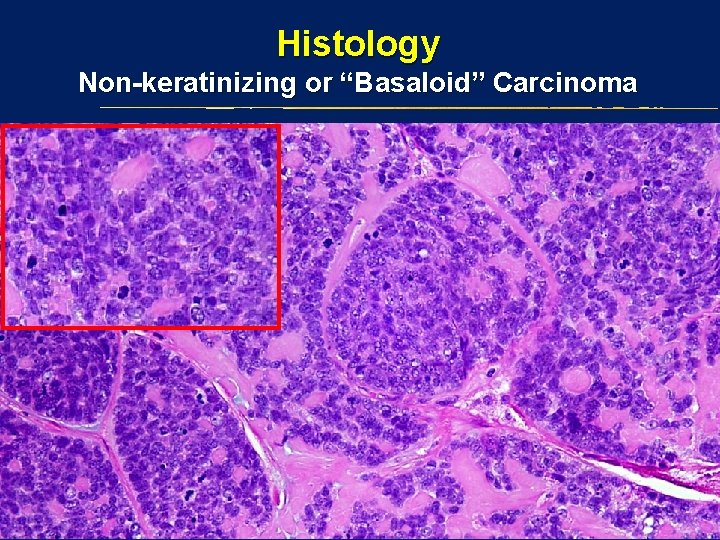
Histology Non-keratinizing or “Basaloid” Carcinoma

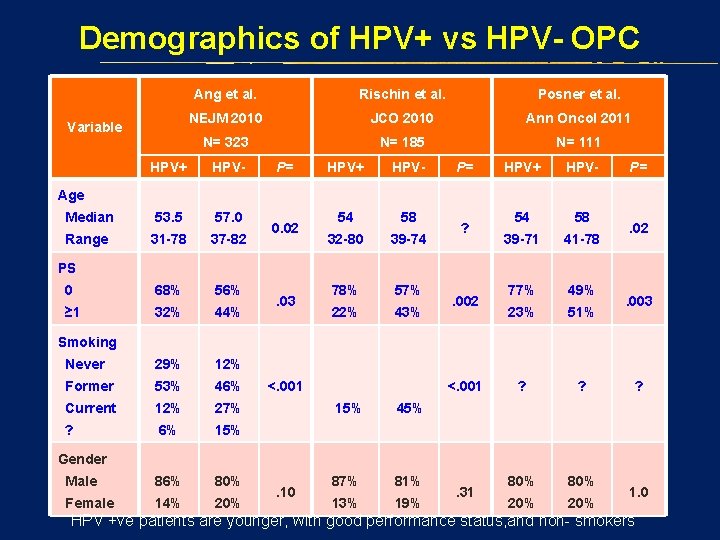
Demographics of HPV+ vs HPV- OPC Variable Ang et al. Rischin et al. Posner et al. NEJM 2010 JCO 2010 Ann Oncol 2011 N= 323 N= 185 N= 111 HPV+ HPV- Median 53. 5 57. 0 Range 31 -78 37 -82 0 68% 56% ≥ 1 32% 44% Never 29% 12% Former 53% 46% Current 12% 27% ? 6% 15% Male 86% 80% Female 14% 20% P= HPV+ HPV- 54 58 32 -80 39 -74 78% 57% 22% 43% P= HPV+ HPV- 54 58 39 -71 41 -78 77% 49% 23% 51% ? ? 80% 20% P= Age 0. 02 ? . 02 PS. 03 . 002 . 003 Smoking <. 001 15% 45% 87% 81% 13% 19% ? Gender. 10 . 31 1. 0 HPV +ve patients are younger, with good performance status, and non- smokers

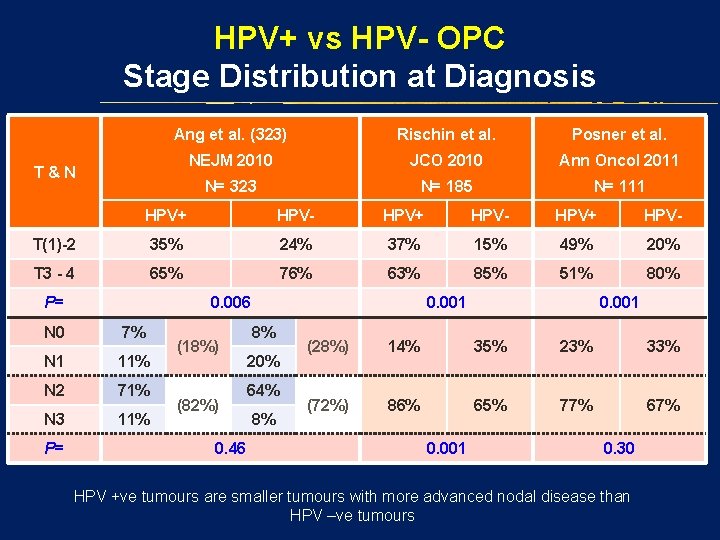
HPV+ vs HPV- OPC Stage Distribution at Diagnosis Ang et al. (323) Rischin et al. Posner et al. NEJM 2010 JCO 2010 Ann Oncol 2011 N= 323 N= 185 N= 111 T&N HPV+ HPV- T(1)-2 35% 24% 37% 15% 49% 20% T 3 - 4 65% 76% 63% 85% 51% 80% P= 0. 006 N 0 7% N 1 11% N 2 71% N 3 11% P= (18%) (82%) 0. 46 0. 001 8% 20% 64% 8% 0. 001 (28%) 14% 35% 23% 33% (72%) 86% 65% 77% 67% 0. 001 0. 30 HPV +ve tumours are smaller tumours with more advanced nodal disease than HPV –ve tumours

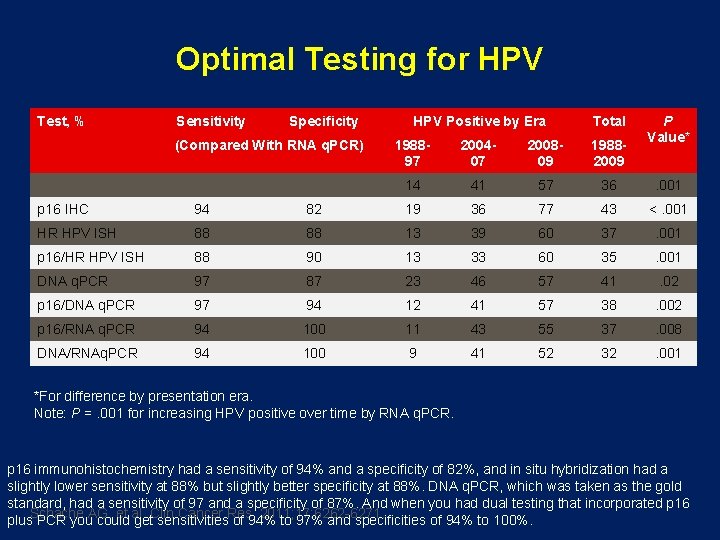
Optimal Testing for HPV Test, % Sensitivity Specificity (Compared With RNA q. PCR) HPV Positive by Era Total P Value* 198897 200407 200809 19882009 14 41 57 36 . 001 p 16 IHC 94 82 19 36 77 43 <. 001 HR HPV ISH 88 88 13 39 60 37 . 001 p 16/HR HPV ISH 88 90 13 33 60 35 . 001 DNA q. PCR 97 87 23 46 57 41 . 02 p 16/DNA q. PCR 97 94 12 41 57 38 . 002 p 16/RNA q. PCR 94 100 11 43 55 37 . 008 DNA/RNAq. PCR 94 100 9 41 52 32 . 001 *For difference by presentation era. Note: P =. 001 for increasing HPV positive over time by RNA q. PCR. p 16 immunohistochemistry had a sensitivity of 94% and a specificity of 82%, and in situ hybridization had a slightly lower sensitivity at 88% but slightly better specificity at 88%. DNA q. PCR, which was taken as the gold standard, had a sensitivity of 97 and a specificity of 87%. And when you had dual testing that incorporated p 16 Schache AG, et al. Clin Cancer Res. 2011; 17: 6262 -6271. plus PCR you could get sensitivities of 94% to 97% and specificities of 94% to 100%.

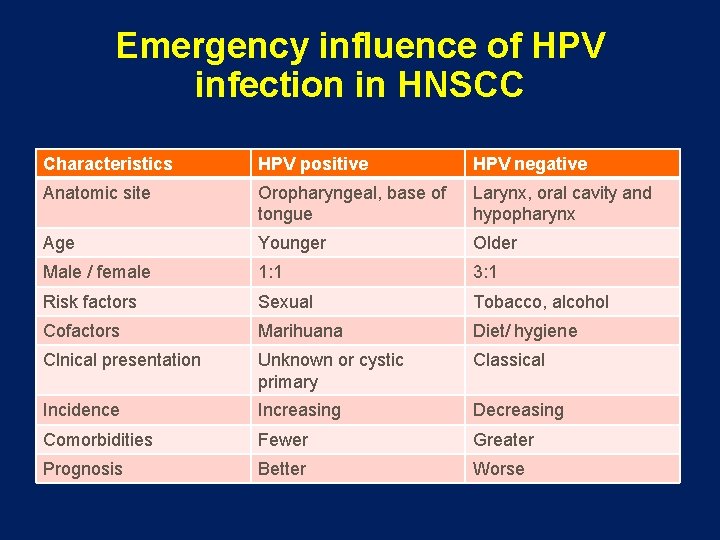
Emergency influence of HPV infection in HNSCC Characteristics HPV positive HPV negative Anatomic site Oropharyngeal, base of tongue Larynx, oral cavity and hypopharynx Age Younger Older Male / female 1: 1 3: 1 Risk factors Sexual Tobacco, alcohol Cofactors Marihuana Diet/ hygiene Clnical presentation Unknown or cystic primary Classical Incidence Increasing Decreasing Comorbidities Fewer Greater Prognosis Better Worse


Prognostic Significance

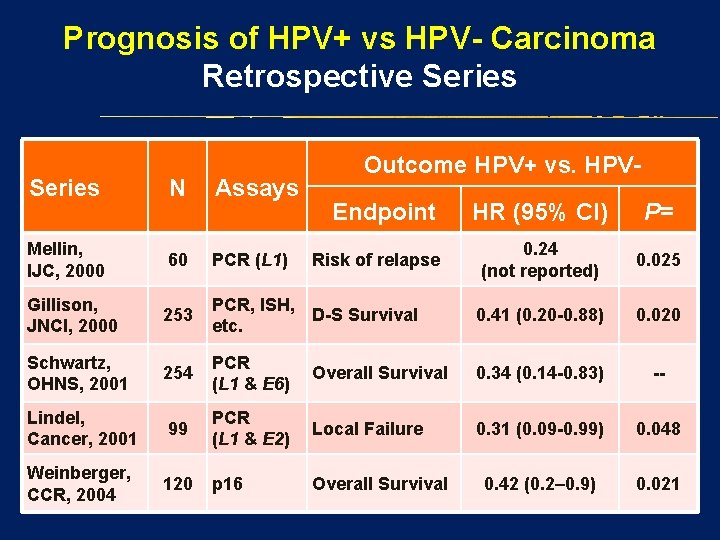
Prognosis of HPV+ vs HPV- Carcinoma Retrospective Series N Assays Mellin, IJC, 2000 60 PCR (L 1) Gillison, JNCI, 2000 253 Schwartz, OHNS, 2001 Outcome HPV+ vs. HPVEndpoint HR (95% CI) P= 0. 24 (not reported) 0. 025 PCR, ISH, D-S Survival etc. 0. 41 (0. 20 -0. 88) 0. 020 254 PCR (L 1 & E 6) Overall Survival 0. 34 (0. 14 -0. 83) -- Lindel, Cancer, 2001 99 PCR (L 1 & E 2) Local Failure 0. 31 (0. 09 -0. 99) 0. 048 Weinberger, CCR, 2004 120 p 16 Overall Survival 0. 42 (0. 2– 0. 9) 0. 021 Risk of relapse

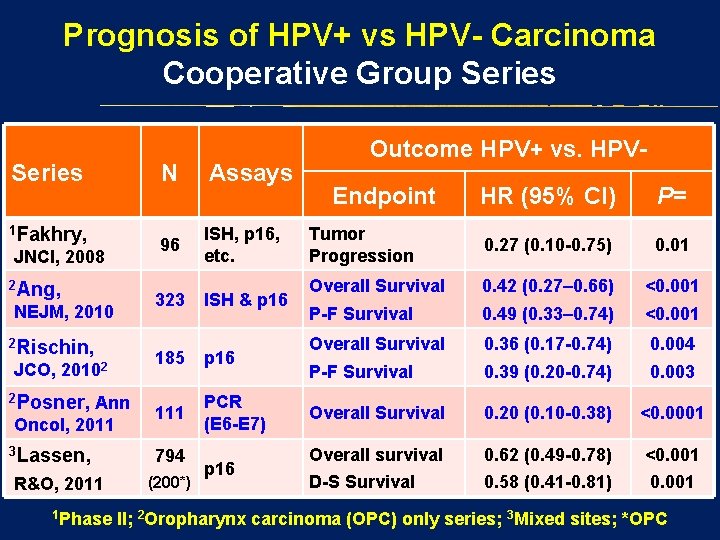
Prognosis of HPV+ vs HPV- Carcinoma Cooperative Group Series 1 Fakhry, JNCI, 2008 2 Ang, NEJM, 2010 2 Rischin, JCO, 20102 2 Posner, Ann Oncol, 2011 3 Lassen, R&O, 2011 1 Phase N Assays 96 ISH, p 16, etc. 323 ISH & p 16 185 p 16 111 PCR (E 6 -E 7) 794 (200*) p 16 Outcome HPV+ vs. HPVEndpoint HR (95% CI) P= Tumor Progression 0. 27 (0. 10 -0. 75) 0. 01 Overall Survival 0. 42 (0. 27– 0. 66) <0. 001 P-F Survival 0. 49 (0. 33– 0. 74) <0. 001 Overall Survival 0. 36 (0. 17 -0. 74) 0. 004 P-F Survival 0. 39 (0. 20 -0. 74) 0. 003 Overall Survival 0. 20 (0. 10 -0. 38) <0. 0001 Overall survival 0. 62 (0. 49 -0. 78) <0. 001 D-S Survival 0. 58 (0. 41 -0. 81) 0. 001 II; 2 Oropharynx carcinoma (OPC) only series; 3 Mixed sites; *OPC

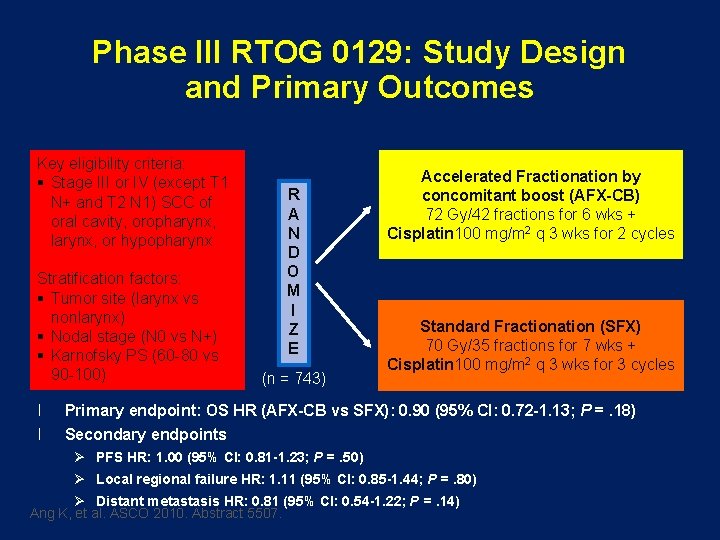
Phase III RTOG 0129: Study Design and Primary Outcomes Key eligibility criteria: § Stage III or IV (except T 1 N+ and T 2 N 1) SCC of oral cavity, oropharynx, larynx, or hypopharynx Stratification factors: § Tumor site (larynx vs nonlarynx) § Nodal stage (N 0 vs N+) § Karnofsky PS (60 -80 vs 90 -100) l l R A N D O M I Z E (n = 743) Accelerated Fractionation by concomitant boost (AFX-CB) 72 Gy/42 fractions for 6 wks + Cisplatin 100 mg/m 2 q 3 wks for 2 cycles Standard Fractionation (SFX) 70 Gy/35 fractions for 7 wks + Cisplatin 100 mg/m 2 q 3 wks for 3 cycles Primary endpoint: OS HR (AFX-CB vs SFX): 0. 90 (95% CI: 0. 72 -1. 13; P =. 18) Secondary endpoints Ø PFS HR: 1. 00 (95% CI: 0. 81 -1. 23; P =. 50) Ø Local regional failure HR: 1. 11 (95% CI: 0. 85 -1. 44; P =. 80) Ø Distant metastasis HR: 0. 81 (95% CI: 0. 54 -1. 22; P =. 14) Ang K, et al. ASCO 2010. Abstract 5507.

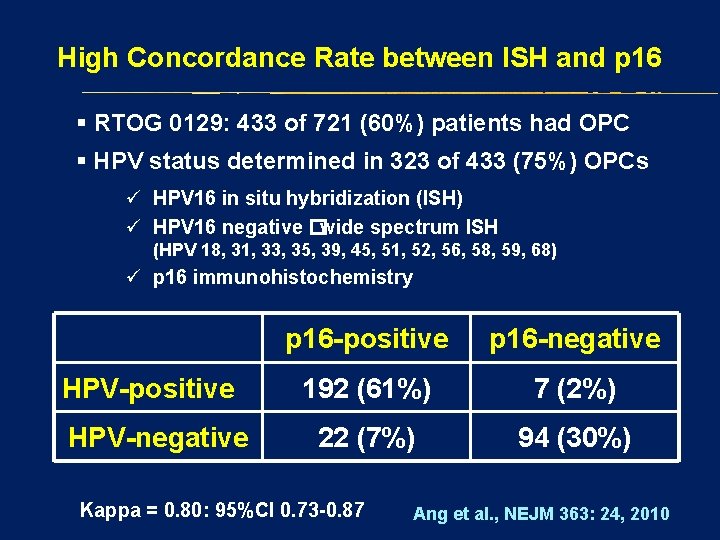
High Concordance Rate between ISH and p 16 § RTOG 0129: 433 of 721 (60%) patients had OPC § HPV status determined in 323 of 433 (75%) OPCs ü HPV 16 in situ hybridization (ISH) ü HPV 16 negative �wide spectrum ISH (HPV 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68) ü p 16 immunohistochemistry HPV-positive HPV-negative p 16 -positive p 16 -negative 192 (61%) 7 (2%) 22 (7%) 94 (30%) Kappa = 0. 80: 95%CI 0. 73 -0. 87 Ang et al. , NEJM 363: 24, 2010

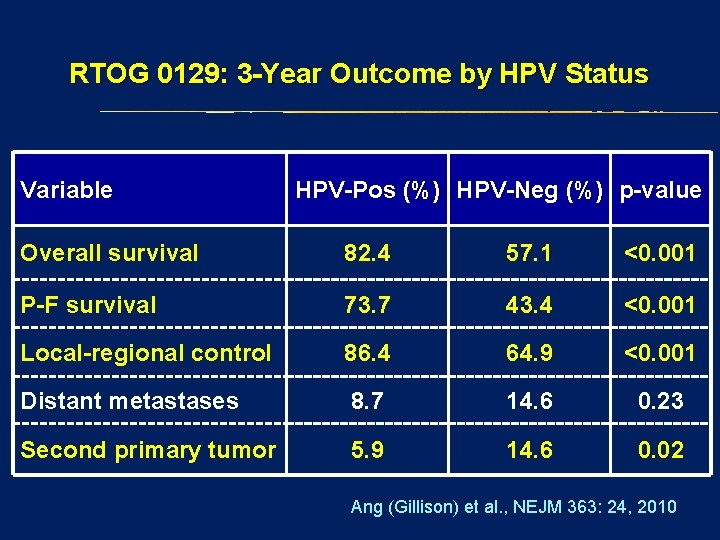
RTOG 0129: 3 -Year Outcome by HPV Status Variable HPV-Pos (%) HPV-Neg (%) p-value Overall survival 82. 4 57. 1 <0. 001 P-F survival 73. 7 43. 4 <0. 001 Local-regional control 86. 4 64. 9 <0. 001 Distant metastases 8. 7 14. 6 0. 23 Second primary tumor 5. 9 14. 6 0. 02 Ang (Gillison) et al. , NEJM 363: 24, 2010

Risk Classification for Overall survival by p-16, Smoking, & T-N Category B Overall Survival A Recursive Partitioning Analysis (RPA)

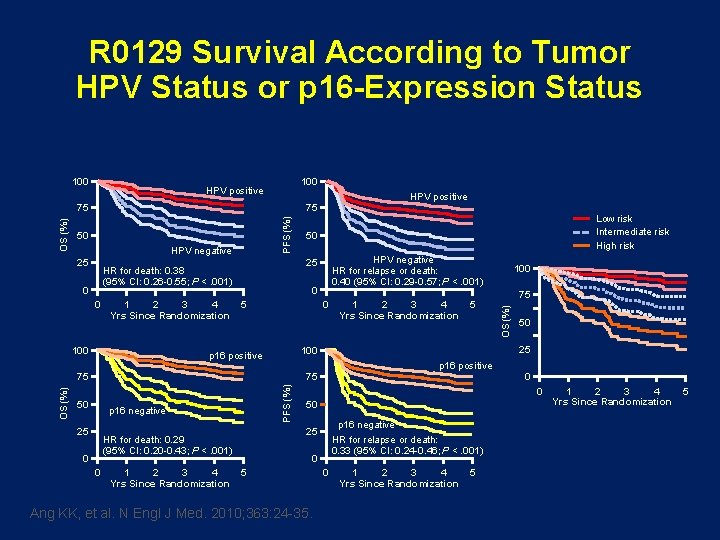
R 0129 Survival According to Tumor HPV Status or p 16 -Expression Status 100 HPV positive 75 50 HPV negative 25 0 1 2 3 4 Yrs Since Randomization 100 HPV negative HR for relapse or death: 0. 40 (95% CI: 0. 29 -0. 57; P <. 001) 0 5 0 PFS (%) p 16 negative 25 0 0 1 2 3 4 Yrs Since Randomization 75 50 p 16 positive 0 0 50 p 16 negative HR for relapse or death: 0. 33 (95% CI: 0. 24 -0. 46; P <. 001) 25 HR for death: 0. 29 (95% CI: 0. 20 -0. 43; P <. 001) 5 100 25 75 50 1 2 3 4 Yrs Since Randomization 100 p 16 positive 75 OS (%) 50 25 HR for death: 0. 38 (95% CI: 0. 26 -0. 55; P <. 001) 0 Low risk Intermediate risk High risk OS (%) PFS (%) OS (%) 75 0 5 Ang KK, et al. N Engl J Med. 2010; 363: 24 -35. 0 1 2 3 4 Yrs Since Randomization 5

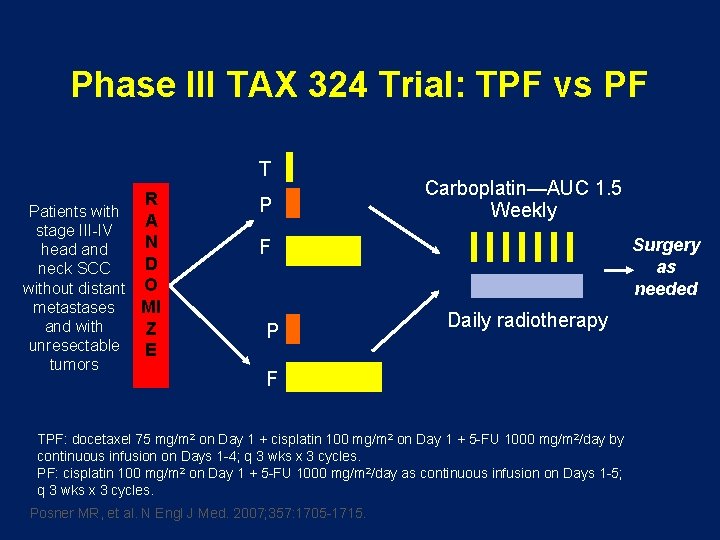
Phase III TAX 324 Trial: TPF vs PF T R Patients with A stage III-IV N head and D neck SCC without distant O metastases MI and with Z unresectable E tumors P Carboplatin—AUC 1. 5 Weekly Surgery as needed F P Daily radiotherapy F TPF: docetaxel 75 mg/m 2 on Day 1 + cisplatin 100 mg/m 2 on Day 1 + 5 -FU 1000 mg/m 2/day by continuous infusion on Days 1 -4; q 3 wks x 3 cycles. PF: cisplatin 100 mg/m 2 on Day 1 + 5 -FU 1000 mg/m 2/day as continuous infusion on Days 1 -5; q 3 wks x 3 cycles. Posner MR, et al. N Engl J Med. 2007; 357: 1705 -1715.

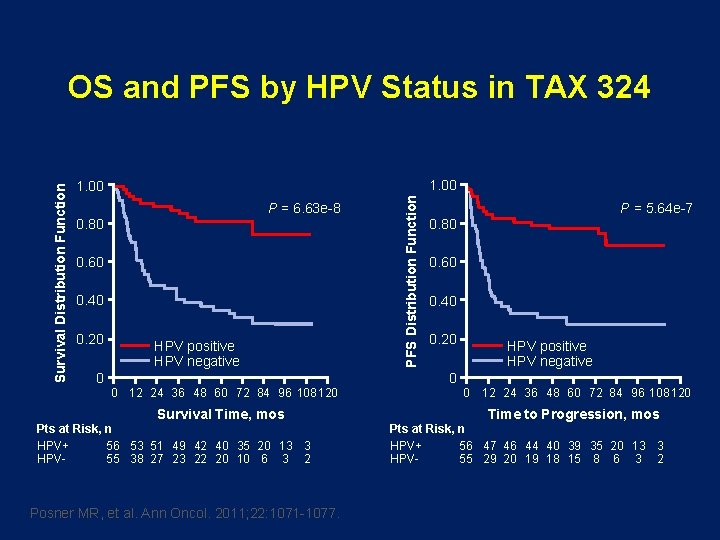
1. 00 P = 6. 63 e-8 0. 80 0. 60 0. 40 0. 20 0 HPV positive HPV negative 0 12 24 36 48 60 72 84 96 108 120 Survival Time, mos Pts at Risk, n HPV+ 56 53 51 49 42 40 35 20 13 3 HPV 55 38 27 23 22 20 10 6 3 2 Posner MR, et al. Ann Oncol. 2011; 22: 1071 -1077. PFS Distribution Function Survival Distribution Function OS and PFS by HPV Status in TAX 324 P = 5. 64 e-7 0. 80 0. 60 0. 40 0. 20 0 HPV positive HPV negative 0 12 24 36 48 60 72 84 96 108 120 Time to Progression, mos Pts at Risk, n HPV+ 56 47 46 44 40 39 35 20 13 3 HPV 55 29 20 19 18 15 8 6 3 2

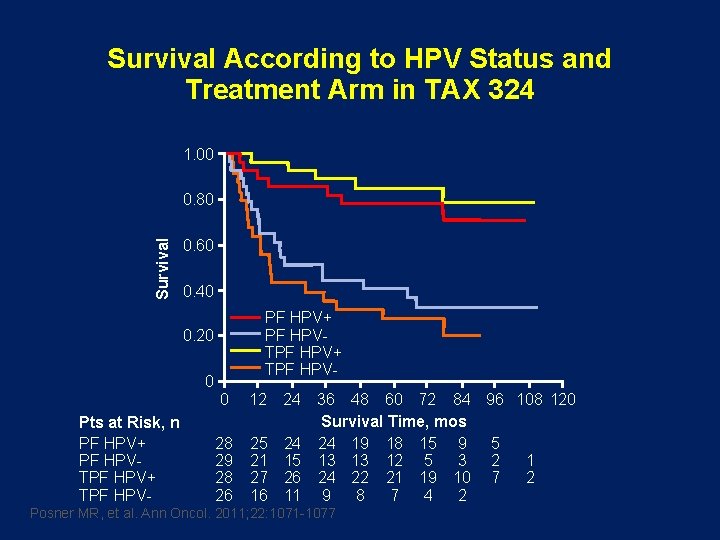
Survival According to HPV Status and Treatment Arm in TAX 324 1. 00 Survival 0. 80 0. 60 0. 40 PF HPV+ PF HPVTPF HPV+ TPF HPV- 0. 20 0 Pts at Risk, n PF HPV+ PF HPVTPF HPV+ TPF HPV- 0 12 28 29 28 26 25 21 27 16 24 36 48 60 72 84 96 108 120 Survival Time, mos 24 24 19 18 15 9 5 15 13 13 12 5 3 2 1 26 24 22 21 19 10 7 2 11 9 8 7 4 2 Posner MR, et al. Ann Oncol. 2011; 22: 1071 -1077

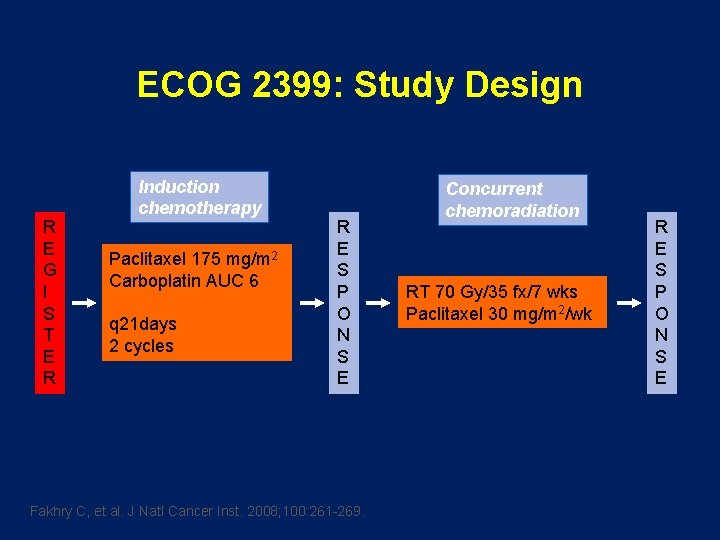
ECOG 2399: Study Design R E G I S T E R Induction chemotherapy Paclitaxel 175 mg/m 2 Carboplatin AUC 6 q 21 days 2 cycles R E S P O N S E Fakhry C, et al. J Natl Cancer Inst. 2008; 100: 261 -269. Concurrent chemoradiation RT 70 Gy/35 fx/7 wks Paclitaxel 30 mg/m 2/wk R E S P O N S E

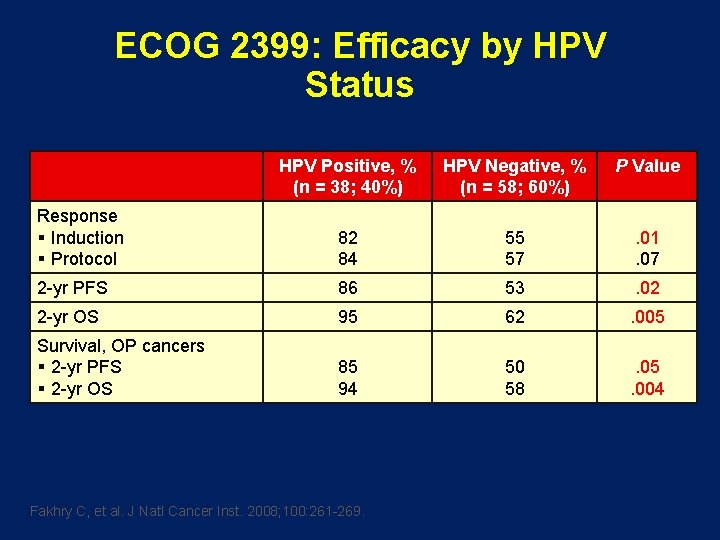
ECOG 2399: Efficacy by HPV Status HPV Positive, % (n = 38; 40%) HPV Negative, % (n = 58; 60%) P Value Response § Induction § Protocol 82 84 55 57 . 01. 07 2 -yr PFS 86 53 . 02 2 -yr OS 95 62 . 005 Survival, OP cancers § 2 -yr PFS § 2 -yr OS 85 94 50 58 . 05. 004 Fakhry C, et al. J Natl Cancer Inst. 2008; 100: 261 -269.

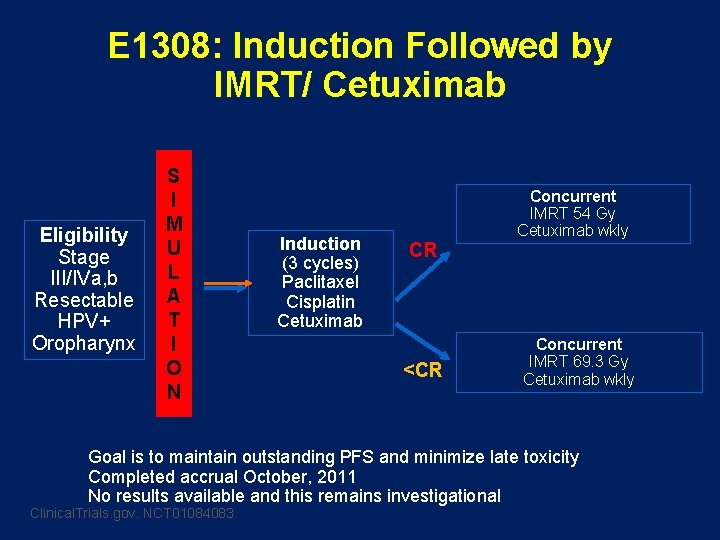
E 1308: Induction Followed by IMRT/ Cetuximab Eligibility Stage III/IVa, b Resectable HPV+ Oropharynx S I M U L A T I O N Induction (3 cycles) Paclitaxel Cisplatin Cetuximab CR <CR Concurrent IMRT 54 Gy Cetuximab wkly Concurrent IMRT 69. 3 Gy Cetuximab wkly Goal is to maintain outstanding PFS and minimize late toxicity Completed accrual October, 2011 No results available and this remains investigational Clinical. Trials. gov. NCT 01084083.

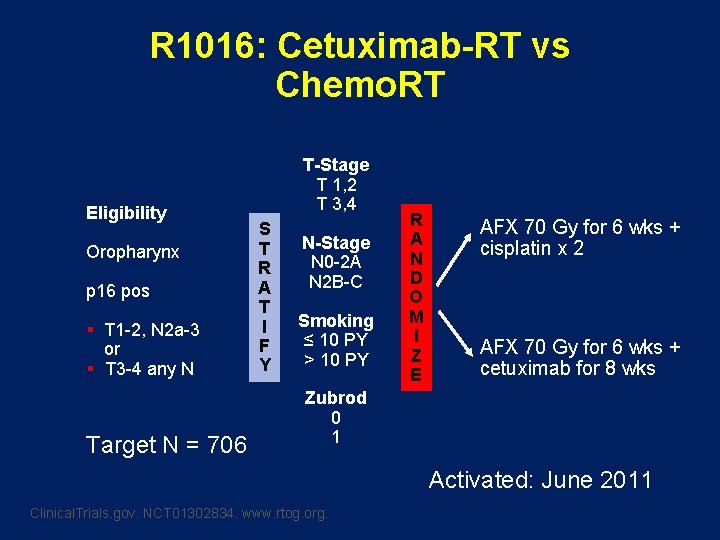
R 1016: Cetuximab-RT vs Chemo. RT Eligibility Oropharynx p 16 pos § T 1 -2, N 2 a-3 or § T 3 -4 any N Target N = 706 T-Stage T 1, 2 T 3, 4 S T R A T I F Y N-Stage N 0 -2 A N 2 B-C Smoking ≤ 10 PY > 10 PY R A N D O M I Z E AFX 70 Gy for 6 wks + cisplatin x 2 AFX 70 Gy for 6 wks + cetuximab for 8 wks Zubrod 0 1 Activated: June 2011 Clinical. Trials. gov. NCT 01302834. www. rtog. org.

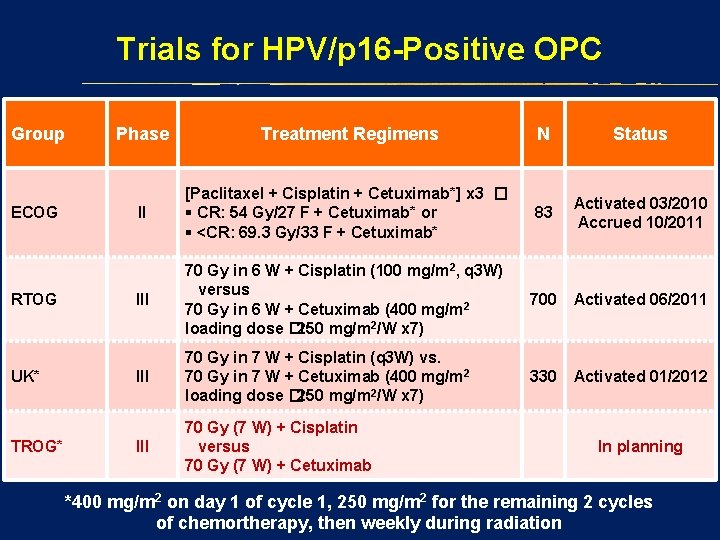
Trials for HPV/p 16 -Positive OPC Group ECOG RTOG UK* TROG* Phase Treatment Regimens N Status II [Paclitaxel + Cisplatin + Cetuximab*] x 3 � § CR: 54 Gy/27 F + Cetuximab* or § <CR: 69. 3 Gy/33 F + Cetuximab* 83 Activated 03/2010 Accrued 10/2011 III 70 Gy in 6 W + Cisplatin (100 mg/m 2, q 3 W) versus 70 Gy in 6 W + Cetuximab (400 mg/m 2 loading dose � 250 mg/m 2/W x 7) 700 Activated 06/2011 III 70 Gy in 7 W + Cisplatin (q 3 W) vs. 70 Gy in 7 W + Cetuximab (400 mg/m 2 loading dose � 250 mg/m 2/W x 7) 330 Activated 01/2012 III 70 Gy (7 W) + Cisplatin versus 70 Gy (7 W) + Cetuximab In planning *400 mg/m 2 on day 1 of cycle 1, 250 mg/m 2 for the remaining 2 cycles of chemortherapy, then weekly during radiation

A french love story?

Up or down TREATMENT

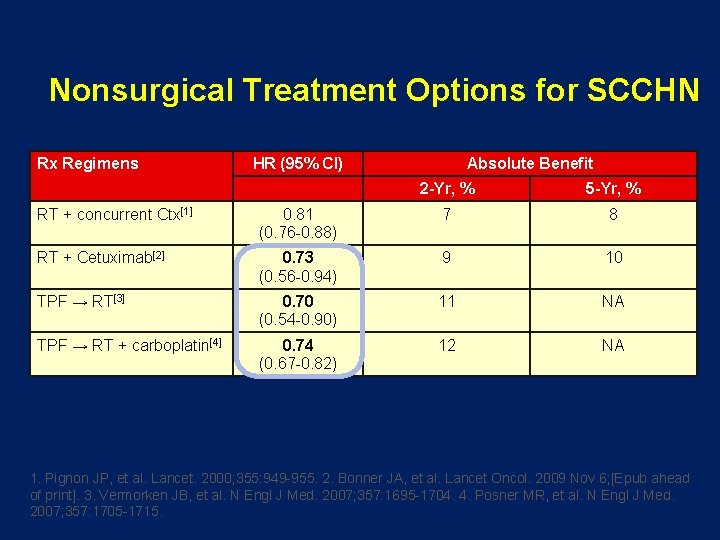
Nonsurgical Treatment Options for SCCHN Rx Regimens HR (95% CI) Absolute Benefit 2 -Yr, % 5 -Yr, % RT + concurrent Ctx[1] 0. 81 (0. 76 -0. 88) 7 8 RT + Cetuximab[2] 0. 73 (0. 56 -0. 94) 9 10 TPF → RT[3] 0. 70 (0. 54 -0. 90) 11 NA TPF → RT + carboplatin[4] 0. 74 (0. 67 -0. 82) 12 NA 1. Pignon JP, et al. Lancet. 2000; 355: 949 -955. 2. Bonner JA, et al. Lancet Oncol. 2009 Nov 6; [Epub ahead of print]. 3. Vermorken JB, et al. N Engl J Med. 2007; 357: 1695 -1704. 4. Posner MR, et al. N Engl J Med. 2007; 357: 1705 -1715.

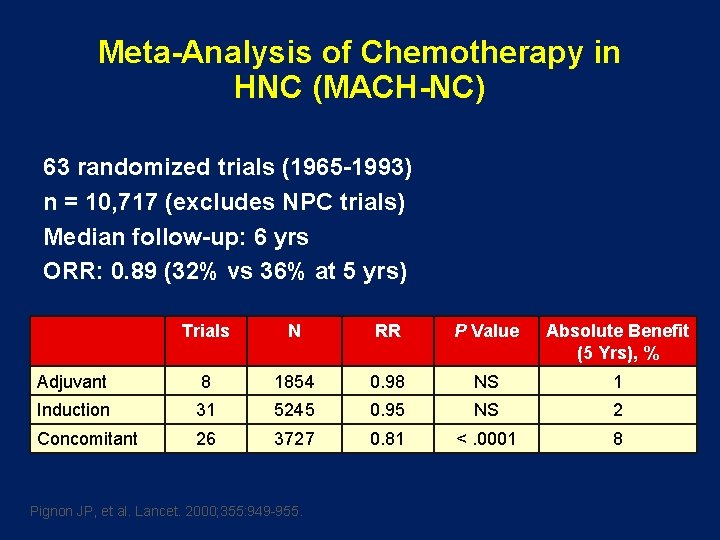
Meta-Analysis of Chemotherapy in HNC (MACH-NC) 63 randomized trials (1965 -1993) n = 10, 717 (excludes NPC trials) Median follow-up: 6 yrs ORR: 0. 89 (32% vs 36% at 5 yrs) Trials N RR P Value Absolute Benefit (5 Yrs), % Adjuvant 8 1854 0. 98 NS 1 Induction 31 5245 0. 95 NS 2 Concomitant 26 3727 0. 81 <. 0001 8 Pignon JP, et al. Lancet. 2000; 355: 949 -955.

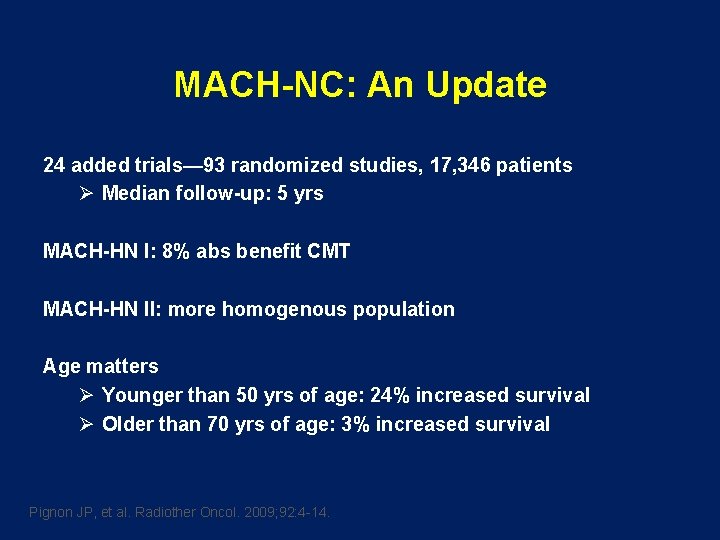
MACH-NC: An Update 24 added trials— 93 randomized studies, 17, 346 patients Ø Median follow-up: 5 yrs MACH-HN I: 8% abs benefit CMT MACH-HN II: more homogenous population Age matters Ø Younger than 50 yrs of age: 24% increased survival Ø Older than 70 yrs of age: 3% increased survival Pignon JP, et al. Radiother Oncol. 2009; 92: 4 -14.

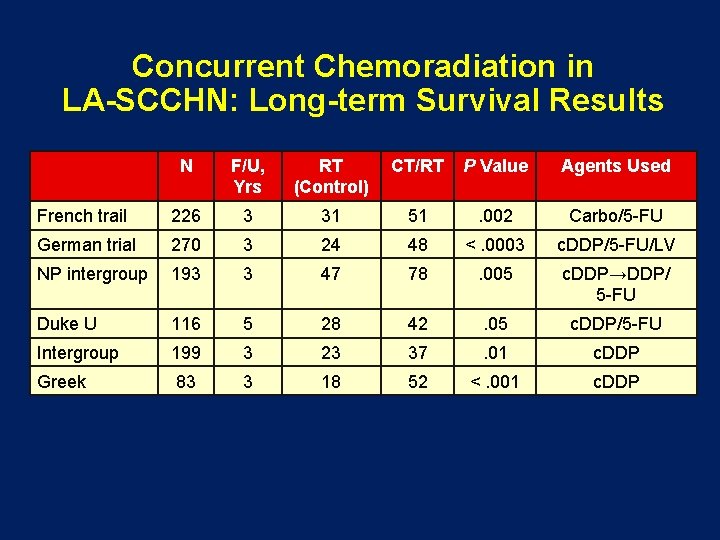
Concurrent Chemoradiation in LA-SCCHN: Long-term Survival Results N F/U, Yrs RT (Control) CT/RT P Value Agents Used French trail 226 3 31 51 . 002 Carbo/5 -FU German trial 270 3 24 48 <. 0003 c. DDP/5 -FU/LV NP intergroup 193 3 47 78 . 005 c. DDP→DDP/ 5 -FU Duke U 116 5 28 42 . 05 c. DDP/5 -FU Intergroup 199 3 23 37 . 01 c. DDP Greek 83 3 18 52 <. 001 c. DDP

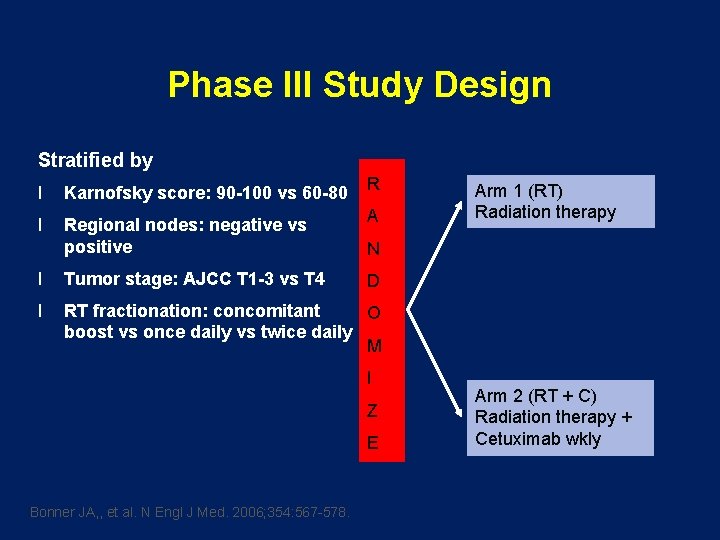
Phase III Study Design Stratified by l Karnofsky score: 90 -100 vs 60 -80 l Regional nodes: negative vs positive R A N l Tumor stage: AJCC T 1 -3 vs T 4 l RT fractionation: concomitant O boost vs once daily vs twice daily M D I Z E Bonner JA, , et al. N Engl J Med. 2006; 354: 567 -578. Arm 1 (RT) Radiation therapy Arm 2 (RT + C) Radiation therapy + Cetuximab wkly

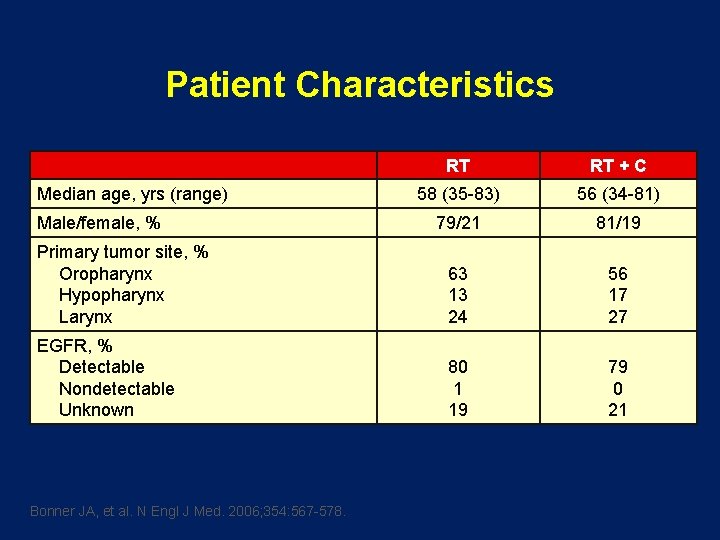
Patient Characteristics RT RT + C 58 (35 -83) 56 (34 -81) 79/21 81/19 Primary tumor site, % Oropharynx Hypopharynx Larynx 63 13 24 56 17 27 EGFR, % Detectable Nondetectable Unknown 80 1 19 79 0 21 Median age, yrs (range) Male/female, % Bonner JA, et al. N Engl J Med. 2006; 354: 567 -578.

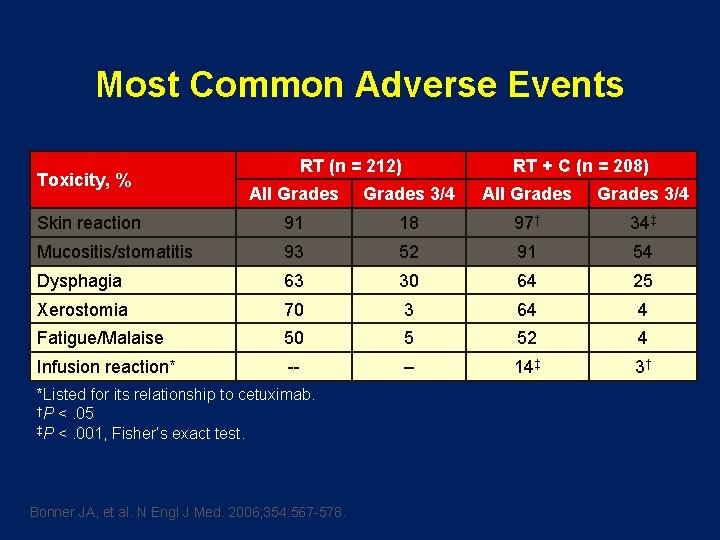
Most Common Adverse Events Toxicity, % RT (n = 212) RT + C (n = 208) All Grades 3/4 Skin reaction 91 18 97† 34‡ Mucositis/stomatitis 93 52 91 54 Dysphagia 63 30 64 25 Xerostomia 70 3 64 4 Fatigue/Malaise 50 5 52 4 Infusion reaction* -- – 14‡ 3† *Listed for its relationship to cetuximab. †P <. 05 ‡P <. 001, Fisher’s exact test. Bonner JA, et al. N Engl J Med. 2006; 354: 567 -578.

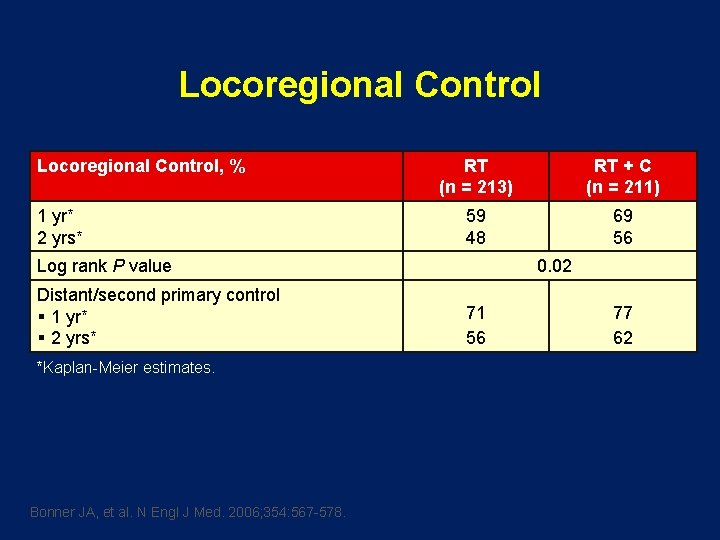
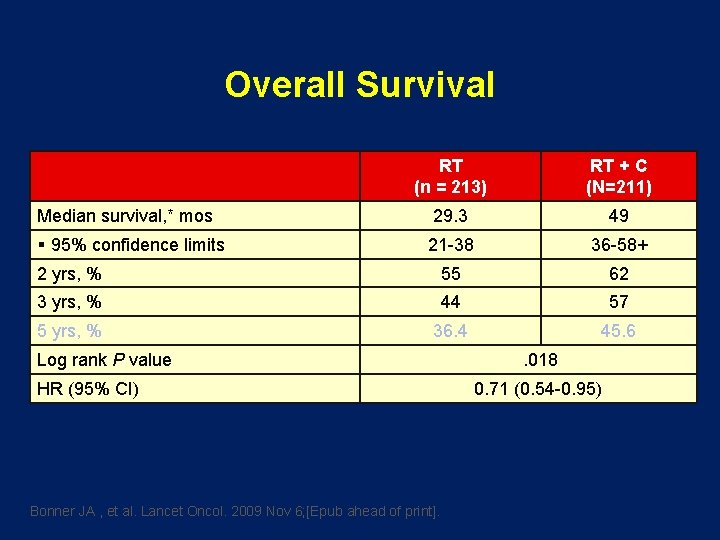
Locoregional Control, % 1 yr* 2 yrs* RT (n = 213) RT + C (n = 211) 59 48 69 56 Log rank P value Distant/second primary control § 1 yr* § 2 yrs* *Kaplan-Meier estimates. Bonner JA, et al. N Engl J Med. 2006; 354: 567 -578. 0. 02 71 56 77 62

Overall Survival RT (n = 213) RT + C (N=211) 29. 3 49 21 -38 36 -58+ 2 yrs, % 55 62 3 yrs, % 44 57 5 yrs, % 36. 4 45. 6 Median survival, * mos § 95% confidence limits Log rank P value HR (95% CI) Bonner JA , et al. Lancet Oncol. 2009 Nov 6; [Epub ahead of print]. . 018 0. 71 (0. 54 -0. 95)

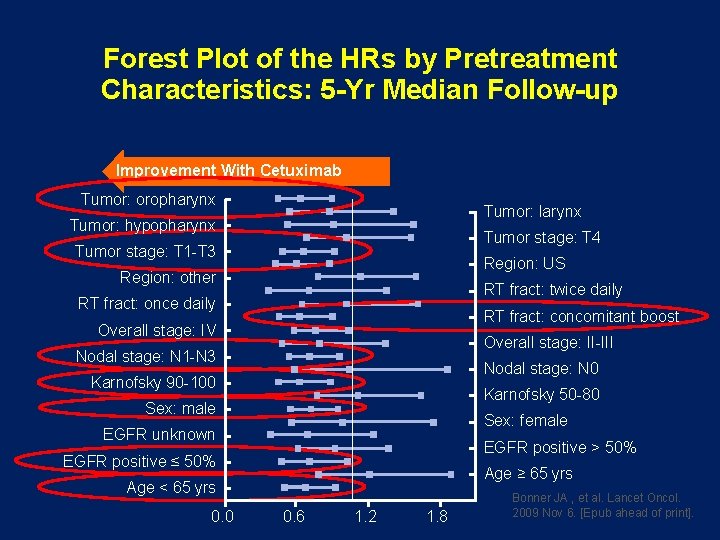
Forest Plot of the HRs by Pretreatment Characteristics: 5 -Yr Median Follow-up Improvement With Cetuximab Tumor: oropharynx Tumor: larynx Tumor: hypopharynx Tumor stage: T 4 Tumor stage: T 1 -T 3 Region: US Region: other RT fract: twice daily RT fract: once daily RT fract: concomitant boost Overall stage: IV Overall stage: II-III Nodal stage: N 1 -N 3 Nodal stage: N 0 Karnofsky 90 -100 Karnofsky 50 -80 Sex: male Sex: female EGFR unknown EGFR positive > 50% EGFR positive ≤ 50% Age ≥ 65 yrs Age < 65 yrs 0. 0 0. 6 1. 2 1. 8 Bonner JA , et al. Lancet Oncol. 2009 Nov 6. [Epub ahead of print].
![TITLE [TITLE]](https://slidetodoc.com/presentation_image_h2/cfb61222c4d5dba96c6073147f5a65a9/image-58.jpg)
[TITLE]
![TITLE [TITLE]](https://slidetodoc.com/presentation_image_h2/cfb61222c4d5dba96c6073147f5a65a9/image-59.jpg)
[TITLE]
![TITLE [TITLE]](https://slidetodoc.com/presentation_image_h2/cfb61222c4d5dba96c6073147f5a65a9/image-60.jpg)
[TITLE]

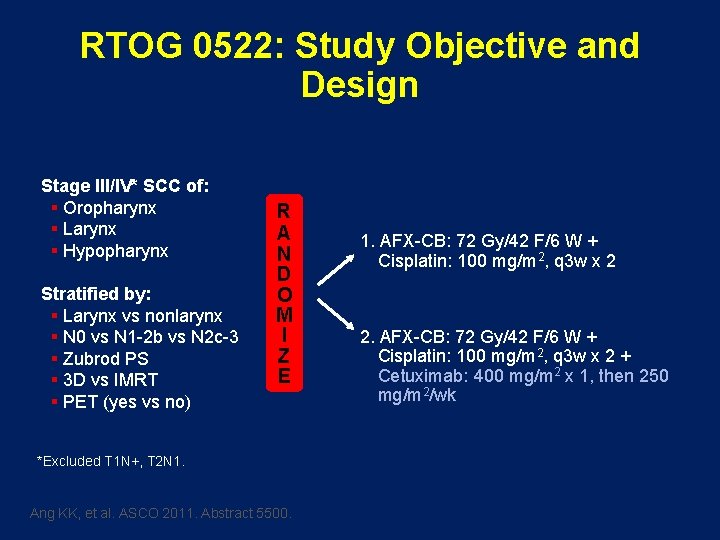
RTOG 0522: Study Objective and Design Stage III/IV* SCC of: § Oropharynx § Larynx § Hypopharynx Stratified by: § Larynx vs nonlarynx § N 0 vs N 1 -2 b vs N 2 c-3 § Zubrod PS § 3 D vs IMRT § PET (yes vs no) R A N D O M I Z E *Excluded T 1 N+, T 2 N 1. Ang KK, et al. ASCO 2011. Abstract 5500. 1. AFX-CB: 72 Gy/42 F/6 W + Cisplatin: 100 mg/m 2, q 3 w x 2 2. AFX-CB: 72 Gy/42 F/6 W + Cisplatin: 100 mg/m 2, q 3 w x 2 + Cetuximab: 400 mg/m 2 x 1, then 250 mg/m 2/wk

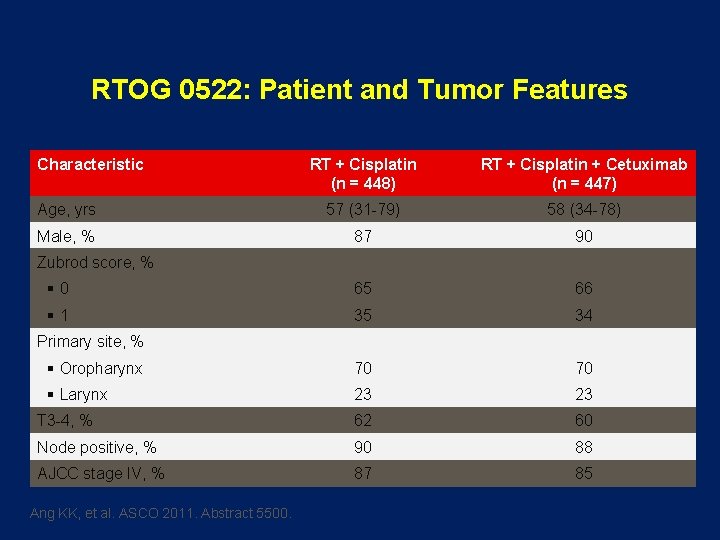
RTOG 0522: Patient and Tumor Features Characteristic RT + Cisplatin (n = 448) RT + Cisplatin + Cetuximab (n = 447) Age, yrs 57 (31 -79) 58 (34 -78) Male, % 87 90 § 0 65 66 § 1 35 34 § Oropharynx 70 70 § Larynx 23 23 T 3 -4, % 62 60 Node positive, % 90 88 AJCC stage IV, % 87 85 Zubrod score, % Primary site, % Ang KK, et al. ASCO 2011. Abstract 5500.

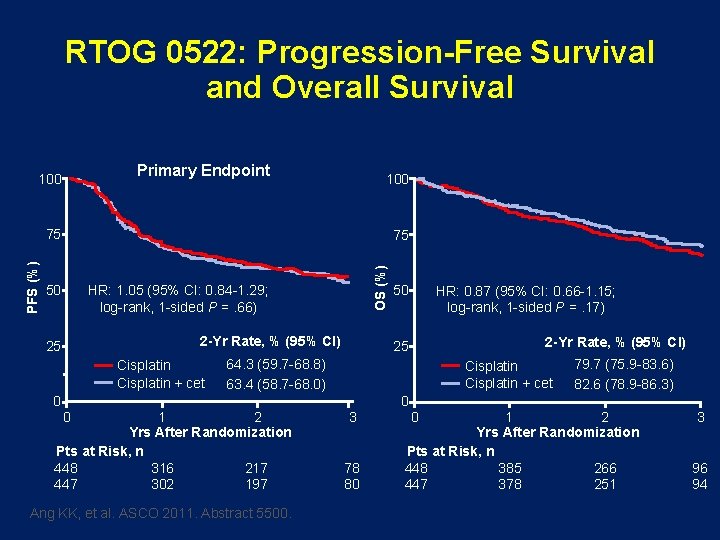
RTOG 0522: Progression-Free Survival and Overall Survival Primary Endpoint 100 75 50 HR: 1. 05 (95% CI: 0. 84 -1. 29; log-rank, 1 -sided P =. 66) 2 -Yr Rate, % (95% CI) 25 Cisplatin + cet 0 OS (%) PFS (%) 75 50 HR: 0. 87 (95% CI: 0. 66 -1. 15; log-rank, 1 -sided P =. 17) 2 -Yr Rate, % (95% CI) 25 64. 3 (59. 7 -68. 8) 63. 4 (58. 7 -68. 0) 0 1 2 Yrs After Randomization Pts at Risk, n 448 316 217 447 302 197 Ang KK, et al. ASCO 2011. Abstract 5500. Cisplatin + cet 3 78 80 0 0 79. 7 (75. 9 -83. 6) 82. 6 (78. 9 -86. 3) 1 2 Yrs After Randomization Pts at Risk, n 448 385 266 447 378 251 3 96 94

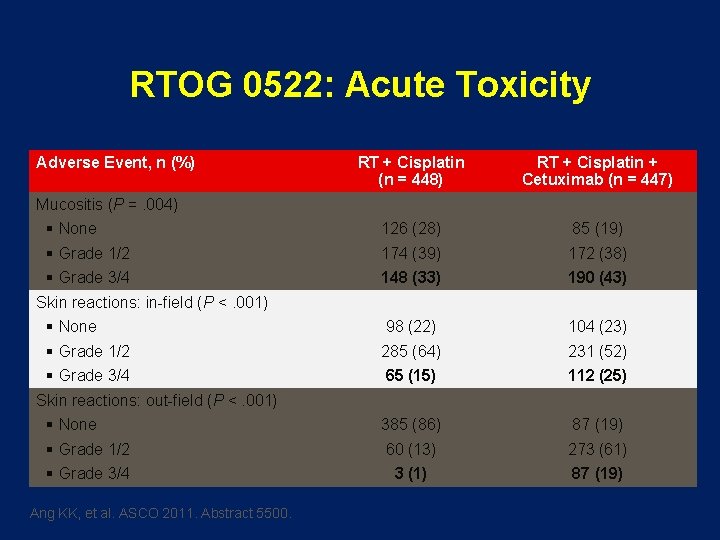
RTOG 0522: Acute Toxicity Adverse Event, n (%) RT + Cisplatin (n = 448) RT + Cisplatin + Cetuximab (n = 447) 126 (28) 85 (19) 174 (39) 172 (38) 148 (33) 190 (43) 98 (22) 104 (23) 285 (64) 231 (52) 65 (15) 112 (25) 385 (86) 87 (19) 60 (13) 273 (61) 3 (1) 87 (19) Mucositis (P =. 004) § None § Grade 1/2 § Grade 3/4 Skin reactions: in-field (P <. 001) § None § Grade 1/2 § Grade 3/4 Skin reactions: out-field (P <. 001) § None § Grade 1/2 § Grade 3/4 Ang KK, et al. ASCO 2011. Abstract 5500.

RTOG 0522: Outcome by p 16 Status and Treatment Regimen l HR < 1 indicates benefit for the cetuximab arm PFS Oropharynx OS ü p 16 -pos (n = 235) ü p 16 -neg (n = 86) ü No specimens (n = 307) Nonoropharynx (n = 267) All patients (N = 895) 0. 0 1. 0 2. 0 0. 0 HR With 95% CI Ang KK, et al. ASCO 2011. Abstract 5500. 1. 0 2. 0

Locoregionally Advanced HNSCC: Summary Chemoradiation remains standard of care High-dose cisplatin preferred in candidates expected to have good tolerability Ø Preference based on relatively extensive study of this agent in this setting In patients requiring substitution with carboplatin (instead of cisplatin), appropriate to add second agent (eg, 5 -FU or paclitaxel) Currently, no evidence to support addition of cetuximab

Other take home mesagges for p 16+ve patients HPV+ had 58% reduction in risk of death HPV+ had a 51% reduction in relapse Three year overall survival HPV+ 83. 6% versus 51. 3% in HPVRisks of death and cancer relapse increased by 1% for each additional pack-year of tobacco smoking

Summary ● HPV-Associated oropharyngeal carcinoma is a distinct head and neck cancer entity ● Prevalence continues to increase for the next 2 decades ● The change in its incidence should be taken into account in interpreting data of completed trials ● Tumor HPV (p 16) status is the strongest prognostic factor identified so far for HNSCC ● Many questions remained to be addressed √ The search for effective, less toxic therapy has become a priority √ How do other molecular changes affect its behavior? ● Therapy should not be changed outside trial setting

A love story with a happy ending…
 Risk factors of head and neck cancer
Risk factors of head and neck cancer There once was a man from tennessee ukulele
There once was a man from tennessee ukulele Tnm 8 head and neck
Tnm 8 head and neck This is home ukulele
This is home ukulele Muscular system head and neck
Muscular system head and neck Muscles of the head neck and shoulders
Muscles of the head neck and shoulders Regional write up head face and neck
Regional write up head face and neck Love love jesus is love god greatest gift lyrics
Love love jesus is love god greatest gift lyrics Eccomyosis
Eccomyosis What has a neck but no head
What has a neck but no head Love is not all: it is not meat nor drink
Love is not all: it is not meat nor drink Indexing head parts
Indexing head parts Negative suction head
Negative suction head Not genuine not true not valid
Not genuine not true not valid The salvation of man is through love and in love
The salvation of man is through love and in love That you must love me and love my dog summary
That you must love me and love my dog summary The attacking firm goes head-to-head with its competitor.
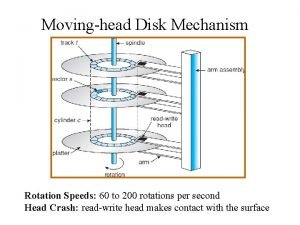
The attacking firm goes head-to-head with its competitor. The head of moving head disk
The head of moving head disk Parts of the chest
Parts of the chest What is the tonic syllable
What is the tonic syllable Biceps femoris innervation
Biceps femoris innervation 7 tones
7 tones The head of moving head disk with 100 tracks
The head of moving head disk with 100 tracks Passionate love vs companionate love
Passionate love vs companionate love Passionate love vs companionate love
Passionate love vs companionate love Infatuation v love
Infatuation v love Infatuated love
Infatuated love Infatuation vs love
Infatuation vs love 2 corinthians 13 4 8
2 corinthians 13 4 8 Consummate love vs companionate love
Consummate love vs companionate love Passionate love vs companionate love
Passionate love vs companionate love Love language test
Love language test Love for the sake of love
Love for the sake of love Your love never fails
Your love never fails Oh my darling i need your love
Oh my darling i need your love Courtly love vs modern love
Courtly love vs modern love Love is his way
Love is his way Nominal character
Nominal character Love begets love do you agree
Love begets love do you agree Let me love and not be respected
Let me love and not be respected Romeo and juliet introduction
Romeo and juliet introduction Rash on upper chest and neck
Rash on upper chest and neck Muscles of facial expression
Muscles of facial expression Neck bulge deepthroat
Neck bulge deepthroat Props in tinikling
Props in tinikling Hand of benediction
Hand of benediction Chapter 24 the forearm, wrist, hand, and, fingers
Chapter 24 the forearm, wrist, hand, and, fingers Halstead ligament
Halstead ligament Boutonniere and swan neck deformity
Boutonniere and swan neck deformity Rheumatoid nodules
Rheumatoid nodules Dorsum of hand
Dorsum of hand Extra articular manifestations of rheumatoid arthritis
Extra articular manifestations of rheumatoid arthritis Boutonniere and swan neck deformity
Boutonniere and swan neck deformity Waiter's tip deformity
Waiter's tip deformity Artrography
Artrography Petit's triangle
Petit's triangle Causal organism of neck and bulb rot in garlic is _____
Causal organism of neck and bulb rot in garlic is _____ Heaven is not closed
Heaven is not closed Head body legs
Head body legs Exposition of the story the gorgon's head
Exposition of the story the gorgon's head Chapter 24 the immune and lymphatic systems and cancer
Chapter 24 the immune and lymphatic systems and cancer Lymph diagram
Lymph diagram Love is not proud
Love is not proud You can find joy in serving god
You can find joy in serving god The joy of serving god
The joy of serving god 1 corinthians 16 22
1 corinthians 16 22 Sonnet 29 edna st vincent millay
Sonnet 29 edna st vincent millay Love is not
Love is not Too broad too narrow
Too broad too narrow