Nordic Clinical Trials and registries in a pediatric
























- Slides: 36

Nordic Clinical Trials and registries in a pediatric setting Thomas Frandsen Consultant, MD, Ph. D, Rigshospitalet Copenhagen May 23 rd 2017

NOPHO


Cancer in children Reasons for death in European Children. Wolfe, Lancet 2013

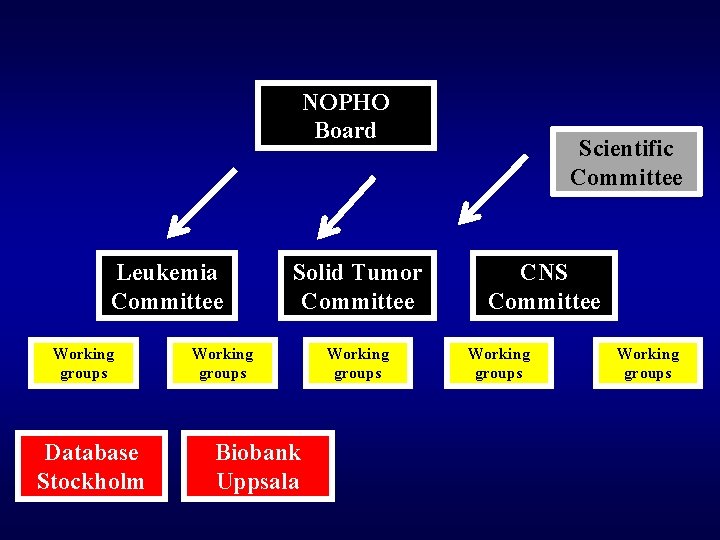
NOPHO Board Leukemia Committee Working groups Database Stockholm Solid Tumor Committee Working groups Biobank Uppsala Working groups Scientific Committee CNS Committee Working groups

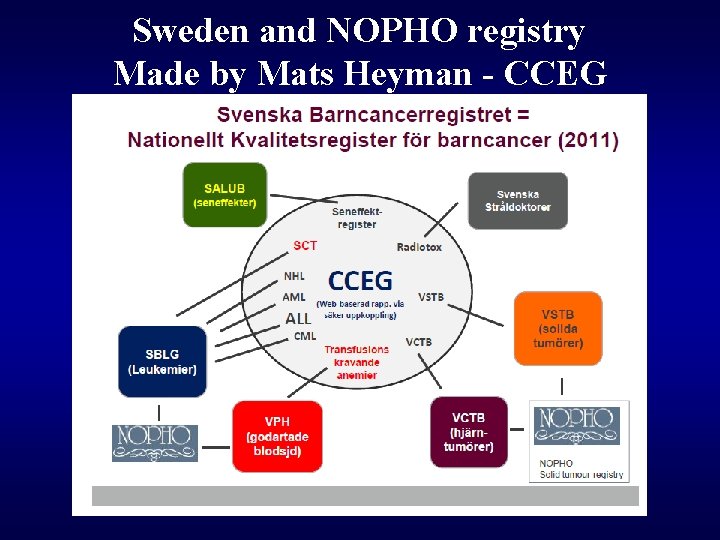
Sweden and NOPHO registry Made by Mats Heyman - CCEG Nordic Cooperation

What is registered – by Mats Heyman - CCEG Nordic Cooperation

7 Countries, 7 languages Population 30 millions 5 million children 200 ALL children per year 30 ALL treatment centres Common NOPHO protocols since 1986

Pediatric Cancer Acute Lymphoblastic Leukemia ALL • Rare disesase • Very little focus from the pharma industry • Central Database • Biobank Nordic Cooperation

Nordic Clinical Trial Challenges • 5 (7) countries, 5 (7) languages • 5 (7) national authorities – Medicines Agencies • 5 (7)National Data protection agencies • 5 (7) Ethical Boards • 5 (7) interpretations of the EU Directive and 5 (7) sets of ethical rules • Strategy and funding for GCP Nordic Cooperation monitoring in Investigator Initiated trials

ALL – mostly in children NOPHO: Hjalgrim & Gustafsson et al (NOPHO) 2003

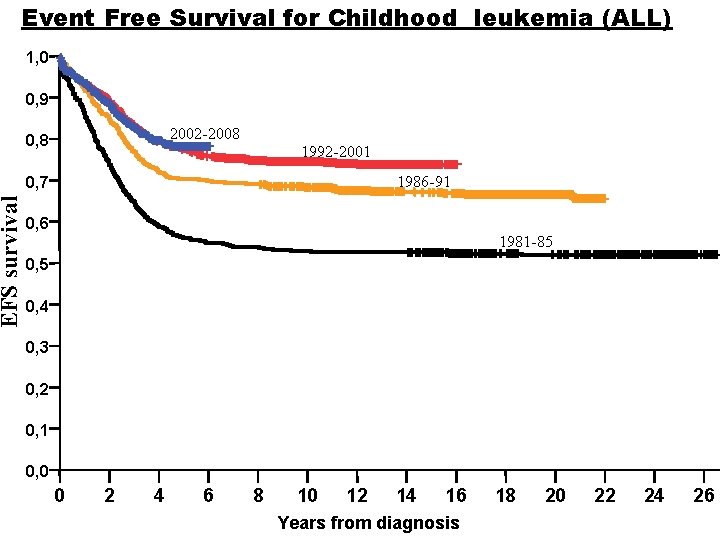
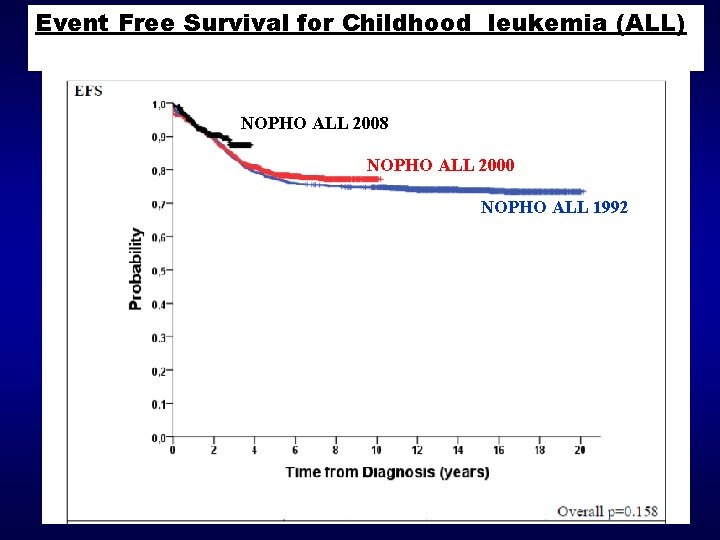
EFS survival Event Free Survival for Childhood leukemia (ALL) 1, 0 0, 9 2002 -2008 0, 8 1992 -2001 1986 -91 0, 7 0, 6 1981 -85 0, 4 0, 3 0, 2 0, 1 0, 0 0 2 4 6 8 10 12 14 16 Years from diagnosis 18 20 22 24 26

NOPHO ALL-2008 Accrual goal (2008 -2016): 1400 children 1. 0 -18 Years 300 children 1. 0 -18 Years 5 Nordic countries (+Rx) 2 Baltic countries (-Rx) 200 ADULTS 18 -45 Years DK, S, N, LT, EE (+F 2014) (-Rx) Biobank For children and adults, identical: Diagnostics (incl. cytogenetics) Risc grouping Common treatment MRD-monitoring Toxicity registration Common platform for research Database EE+ Study Centre Nordic Cooperation LT+

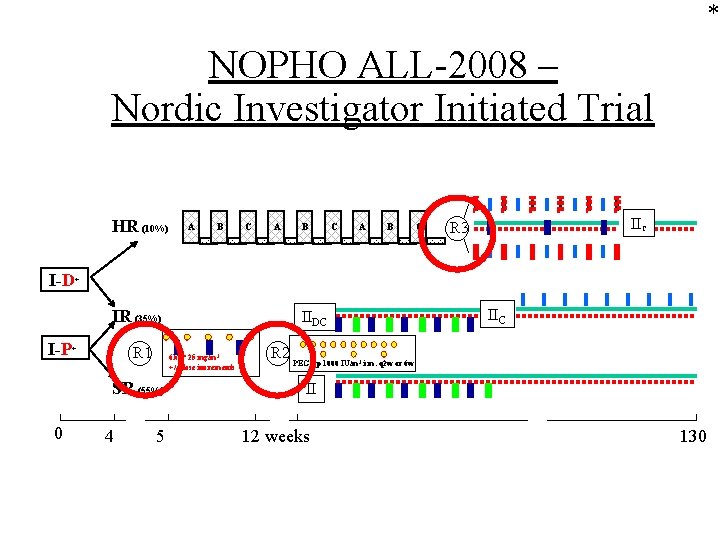
* NOPHO ALL-2008 – Nordic Investigator Initiated Trial HR (10%) A B C IIC R 3 I-D+ IR (35%) I-P+ R 1 6 MP 25 mg/m 2 +/- dose increments SR (55%) 0 4 5 IIC IIDC R 2 PEGasp 1000 IU/m i. m. q 2 w or 6 w 2 II 12 weeks Nordic Cooperation 130

Children are not just small adults Nordic Cooperation

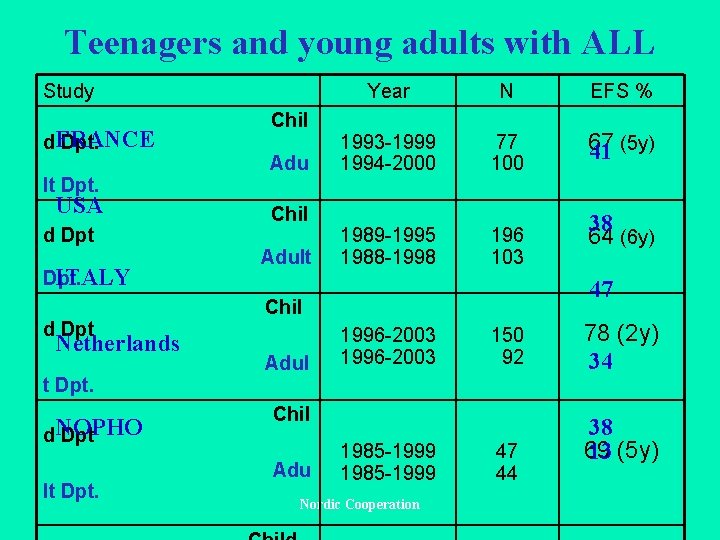
Teenagers and young adults with ALL Study d. FRANCE Dpt. lt Dpt. USA d Dpt ITALY Dpt. d Dpt Netherlands t Dpt. d. NOPHO Dpt lt Dpt. Chil Adult Year N EFS % 1993 -1999 1994 -2000 77 100 67 41 (5 y) 1989 -1995 1988 -1998 196 103 47 Chil Adul 1996 -2003 150 92 Chil Adu 38 64 (6 y) 1985 -1999 Nordic Cooperation 47 44 78 (2 y) 34 38 69 13 (5 y)

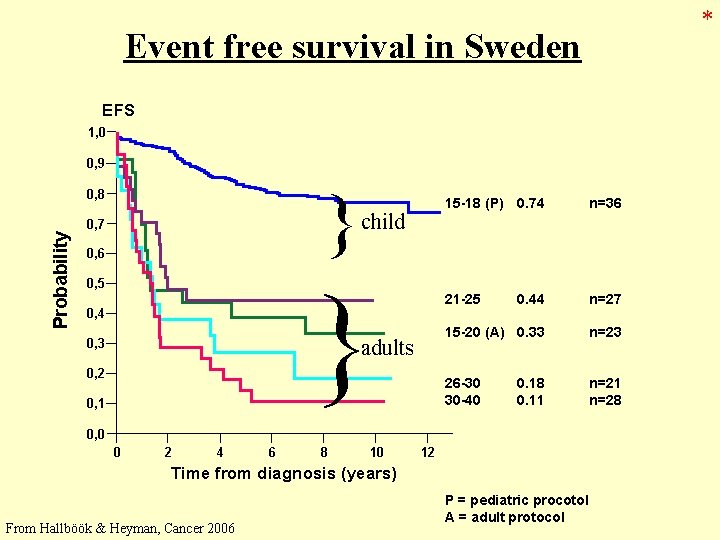
* Event free survival in Sweden EFS 1, 0 0, 9 } Probability 0, 8 0, 7 0, 6 child } 0, 5 0, 4 adults 0, 3 0, 2 0, 1 15 -18 (P) 0. 74 n=36 21 -25 0. 44 n=27 15 -20 (A) 0. 33 n=23 26 -30 30 -40 n=21 n=28 0. 11 0, 0 0 2 4 6 8 10 12 Time from diagnosis (years) Nordic Cooperation From Hallböök & Heyman, Cancer 2006 P = pediatric procotol A = adult protocol

Event Free Survival for Childhood leukemia (ALL) NOPHO ALL 2008 NOPHO ALL 2000 NOPHO ALL 1992 DPS årsmøde 2012

Strategy for the trial • Simple, on-line dataregistration, including SAEs, Death and SUSAR’s • Exclusion of known AE’s • Continuous monitoring of entered data by the study centre. Errors are picked up within a short period. • Nordic GCP network • Help-desk Nordic Cooperation

Strategy of monitoring and registration

NOPHO ALL 2008, children 1. 0 -17. 9, p. EFS March 2012 SR, 0. 96 IR, 0. 88 Event-free survival HR+SCT, 0. 72 HR, 0. 66 Nordic Cooperation Years from diagnosis

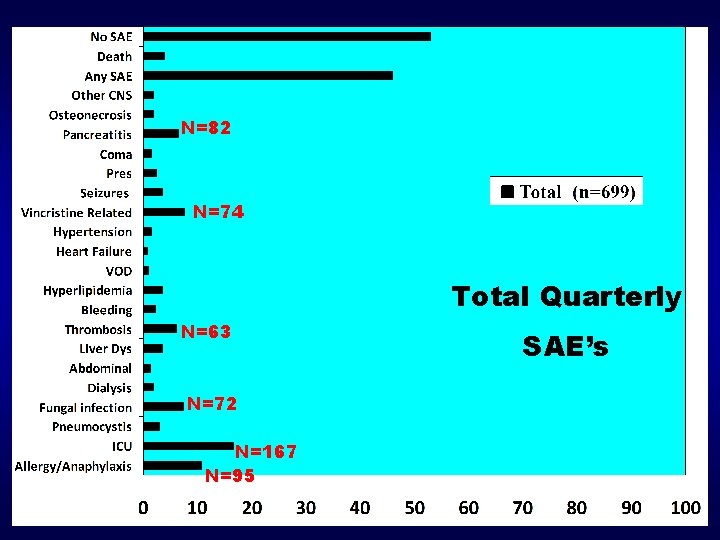
N=82 N=74 Total Quarterly N=63 SAE’s N=72 N=167 N=95 Nordic Cooperation

Total Quarterly SAE’s Age divided Nordic Cooperation

Strategy for the trial • Simple, on-line dataregistration, including SAEs • Exclusion of known AE’s • Continuous monitoring of registered data by the study centre. Errors are picked up within a short period. • Nordic GCP network • Help-desk Nordic Cooperation

AEs not to be reported • a number of toxicities are so well-known and frequent during therapy that they will not be reported. These includes: • For the 6 MP increment study, the following will not be AE-reported: • Since leukopenia is the target toxicity (monitoring parameter), this side-effect will not be regarded as a SAE. This also includes febrile neutropenia leading to hospitalisation or prolongation of ongoing hospitalisation if the patients condition otherwise is good with no signs of septic shock. Since thrombocytopenia is the target toxicity (monitoring parameter) this side-effect will not be regarded as a SAE. A rise in aminotransferases with normal liver function tests (i. e. bilirubin and INR (or coagulation factor 2 -7 -10) is a well-known side effect of HD-MTX and 6 MP and will not be regarded as a SAE, unless in combination with 19. 3. 1. 8. A rise in bilirubin to less than 5 x UNL. • • A fall in coagulation factors, unless in combination with 19. 3. 1. 8. Less than a grade 4 rise in amylase (>5 x UNL, if measured) will not be reported. Kidney dysfunction is a well-known side effect of HD-MTX and will not be regarded as SAE unless it requires dialysis or leads to a permanent kidney dysfunction with s-creatinine >UNL. Stomatitis and dyspepsia with or without liver toxicity are a well-known side effects of HD -MTX and will not be regarded as SAE. Infection/fever leading to hospitalisation or prolongation of existing hospitalisation. Nordic Cooperation

Strategy for the trial • Simple, on-line dataregistration, including SAEs • Exclusion of known AE’s • E-CRF’s in same database • Continuous monitoring of entered data by the study centre. Errors are picked up within a short period. • Nordic GCP network • Help-desk Nordic Cooperation

Strategy for the trial • Simple, on-line dataregistration, including SAEs • Exclusion of known AE’s • E-CRF’s in same database • Continuous monitoring of entered data by the study centre. Errors are picked up within a short period. • Nordic GCP network • Help-desk Nordic Cooperation

Strategy for the trial • Simple, on-line dataregistration, including SAEs • Exclusion of known AE’s • E-CRF’s in same database • Continuous monitoring of entered data by the study centre. Errors are picked up within a short period. • Nordic GCP network • Help-desk Nordic Cooperation

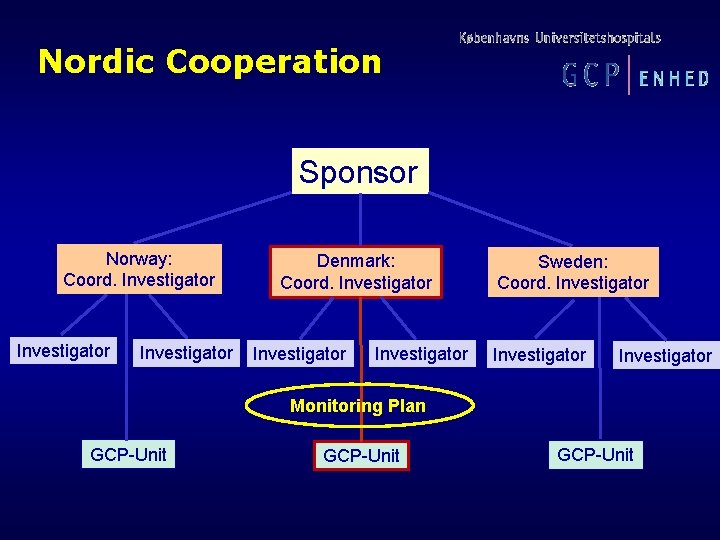
Nordic Cooperation Sponsor Norway: Coord. Investigator Denmark: Coord. Investigator Sweden: Coord. Investigator Monitoring Plan GCP-Unit

Strategy for the trial • Simple, on-line dataregistration, including SAEs • Exclusion of known AE’s • Continuous monitoring of data by the study centre. Errors are picked up within a short period. • Nordic GCP network • On-line Help-desk Nordic Cooperation

Nordic Cooperation

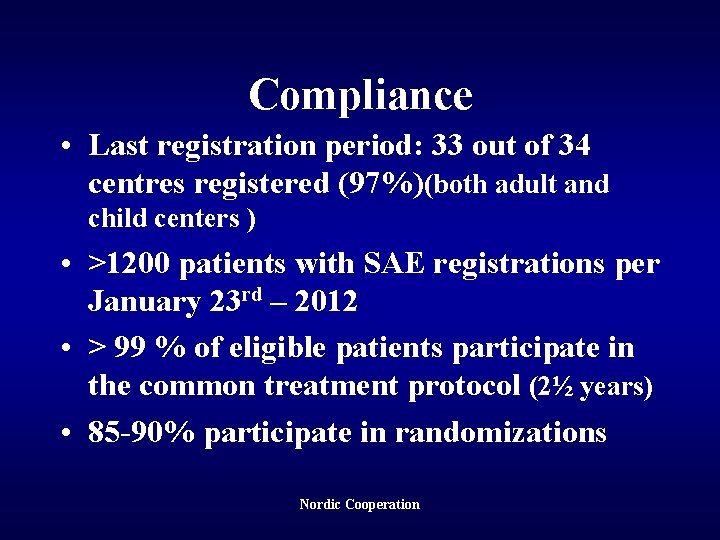
Compliance • Last registration period: 33 out of 34 centres registered (97%)(both adult and child centers ) • >1200 patients with SAE registrations per January 23 rd – 2012 • > 99 % of eligible patients participate in the common treatment protocol (2½ years) • 85 -90% participate in randomizations Nordic Cooperation

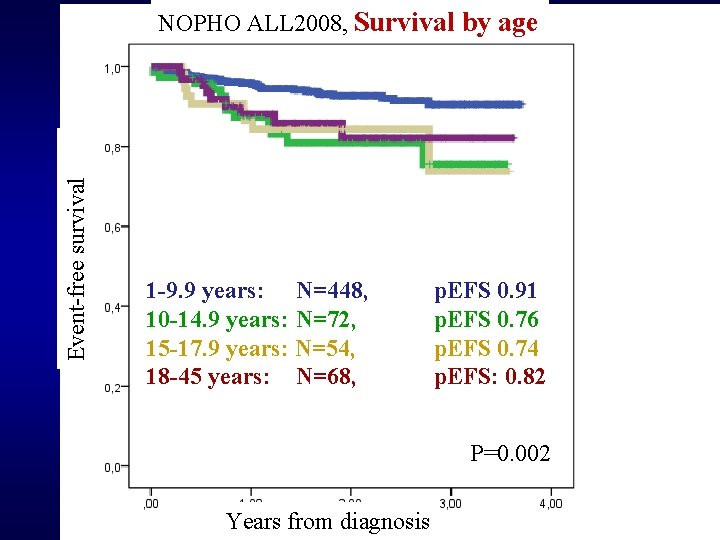
Event-free survival NOPHO ALL 2008, Survival by age 1 -9. 9 years: N=448, 10 -14. 9 years: N=72, 15 -17. 9 years: N=54, 18 -45 years: N=68, p. EFS 0. 91 p. EFS 0. 76 p. EFS 0. 74 p. EFS: 0. 82 P=0. 002 Nordic Cooperation Years from diagnosis

Perhaps adults are just big kids Nordic Cooperation

Biggest challenge in the Nordic Pediatric Oncology setting: Ethical applications – not registries • One entry Or perhaps • VHP- like ethical application Nordic Cooperation

Nordic Cooperation
 Pediatric trials network
Pediatric trials network Nurse registry california
Nurse registry california Phs human subjects and clinical trials information
Phs human subjects and clinical trials information Audits and inspections of clinical trials
Audits and inspections of clinical trials Nida clinical trial network
Nida clinical trial network Site initiation visit ppt
Site initiation visit ppt Clinical trial api
Clinical trial api Clinical research statistician
Clinical research statistician Andrew nunn
Andrew nunn Stratified randomization
Stratified randomization Mpn clinical trials
Mpn clinical trials Hawk irb
Hawk irb Clinical trials
Clinical trials Clinical trials quality by design
Clinical trials quality by design Professor claire harrison
Professor claire harrison Dhl bishkek
Dhl bishkek Clinical hysteria salem witch trials
Clinical hysteria salem witch trials Readyset ohsu
Readyset ohsu Clinical trial prs
Clinical trial prs Clinical trials.gov login
Clinical trials.gov login Pharmaceutical iwr applications
Pharmaceutical iwr applications York trials unit
York trials unit Nordic clinical
Nordic clinical Pediatric clinical nurse specialist programs
Pediatric clinical nurse specialist programs Do we our life done
Do we our life done Design and analysis of cross over trials
Design and analysis of cross over trials British isles and nordic nations map
British isles and nordic nations map British isles and nordic nations map
British isles and nordic nations map European and nordic studies
European and nordic studies Nordic finance and the good society
Nordic finance and the good society British isles and nordic nations map
British isles and nordic nations map Salem witch trials discovery education
Salem witch trials discovery education National geographic salem witch trials
National geographic salem witch trials Malta football trials
Malta football trials Bernoulli trials formula
Bernoulli trials formula Hercules wife
Hercules wife Futuresearch trials
Futuresearch trials