NONPRESCRIPTION NICOTINE REPLACEMENT THERAPY CIGARETTE SMOKING is the













































- Slides: 65

NONPRESCRIPTION NICOTINE REPLACEMENT THERAPY

“CIGARETTE SMOKING… is the chief, single, avoidable cause of death in our society and the most important public health issue of our time. ” C. Everett Koop, M. D. , former U. S. Surgeon General

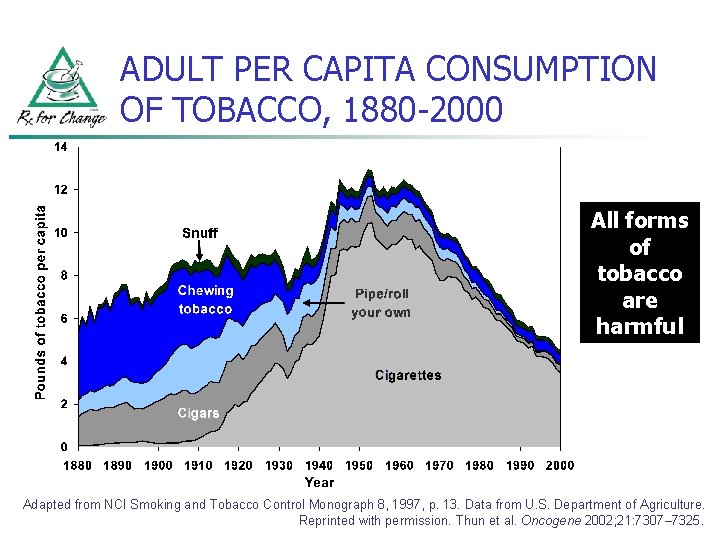
ADULT PER CAPITA CONSUMPTION OF TOBACCO, 1880 -2000 All forms of tobacco are harmful Adapted from NCI Smoking and Tobacco Control Monograph 8, 1997, p. 13. Data from U. S. Department of Agriculture. Reprinted with permission. Thun et al. Oncogene 2002; 21: 7307– 7325.

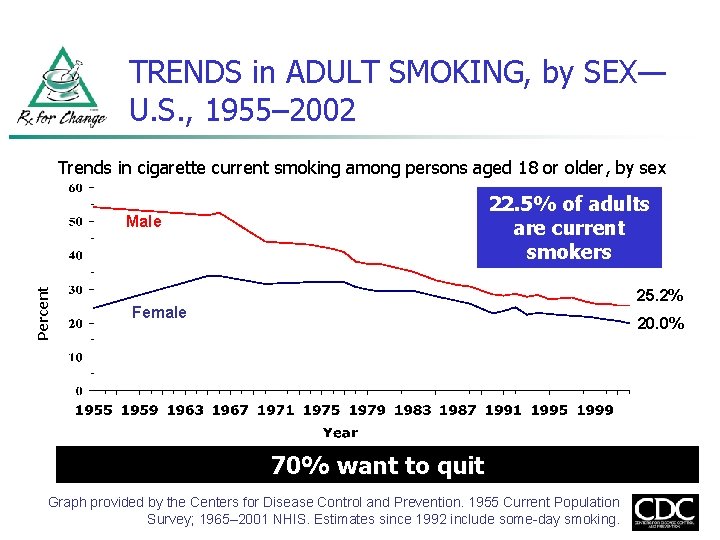
TRENDS in ADULT SMOKING, by SEX— U. S. , 1955– 2002 Trends in cigarette current smoking among persons aged 18 or older, by sex 22. 5% of adults are current smokers Percent Male 25. 2% Female 20. 0% 70% want to quit Graph provided by the Centers for Disease Control and Prevention. 1955 Current Population Survey; 1965– 2001 NHIS. Estimates since 1992 include some-day smoking.

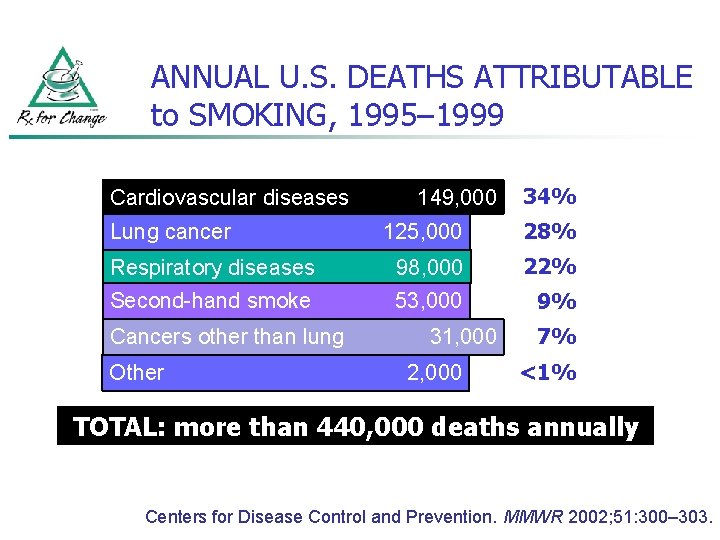
ANNUAL U. S. DEATHS ATTRIBUTABLE to SMOKING, 1995– 1999 Cardiovascular diseases 149, 000 34% 125, 000 28% Respiratory diseases 98, 000 22% Second-hand smoke 53, 000 9% Lung cancer Cancers other than lung Other 31, 000 2, 000 7% <1% TOTAL: more than 440, 000 deaths annually Centers for Disease Control and Prevention. MMWR 2002; 51: 300– 303.

2004 REPORT of the SURGEON GENERAL FOUR MAJOR CONCLUSIONS n n Smoking harms nearly every organ of the body, causing many diseases and reducing the health of smokers in general. Quitting smoking has immediate as well as long-term benefits, reducing risks for diseases caused by smoking and improving health in general. Smoking cigarettes with lower machine-measured yields of tar and nicotine provides no clear benefit to health. Numerous diseases are caused by smoking. U. S. Department of Health and Human Services. The Health Consequences of Smoking: A Report of the Surgeon General, 2004.

HEALTH CONSEQUENCES of SMOKING n Cancers n n n n Lung Laryngeal, pharyngeal, oral cavity, esophagus Pancreatic Bladder and kidney Cervical and endometrial Gastric Acute myeloid leukemia Reduce fertility in women, poor pregnancy outcomes, low birth weight babies, sudden infant death syndrome n Cardiovascular diseases n n n Subclinical atherosclerosis Coronary heart disease Stroke Abdominal aortic aneurysm Respiratory diseases n n Acute respiratory illnesses, e. g. , pneumonia Chronic respiratory diseases, e. g. , COPD n Cataract n Periodontitis U. S. Department of Health and Human Services. The Health Consequences of Smoking: A Report of the Surgeon General, 2004.

CAUSALLY ASSOCIATED HEALTH RISKS of SECOND-HAND SMOKE n Developmental effects n n Carcinogenic effects n n Lung cancer, nasal sinus cancer Cardiovascular effects n n Fetal growth retardation, SIDS EVEN A LITTLE SECOND-HAND SMOKE IS DANGEROUS Heart disease mortality, acute and chronic CHD morbidity Respiratory effects n n Children: acute lower respiratory tract infections, asthma induction and exacerbation, chronic respiratory symptoms, middle ear infections Adults: eye and nasal irritation National Cancer Institute. Health Effects of Exposure to Environmental Tobacco Smoke: The Report of the California Environmental Protection Agency , 1999.

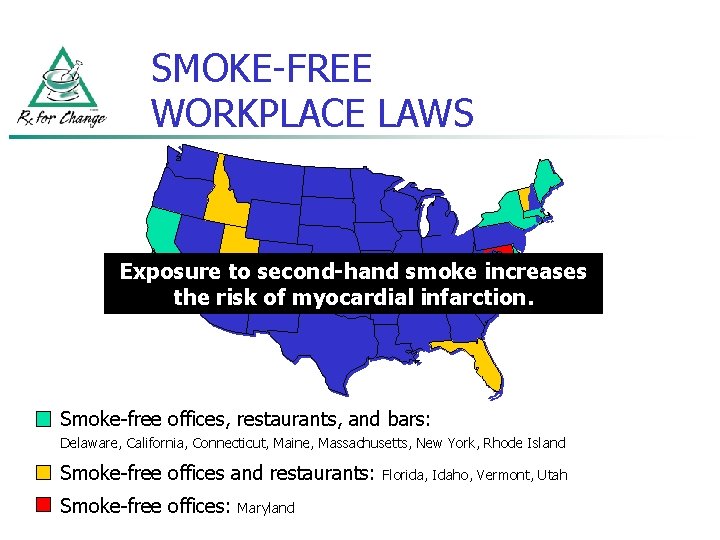
SMOKE-FREE WORKPLACE LAWS Exposure to second-hand smoke increases the risk of myocardial infarction. Smoke-free offices, restaurants, and bars: Delaware, California, Connecticut, Maine, Massachusetts, New York, Rhode Island Smoke-free offices and restaurants: Smoke-free offices: Maryland Florida, Idaho, Vermont, Utah

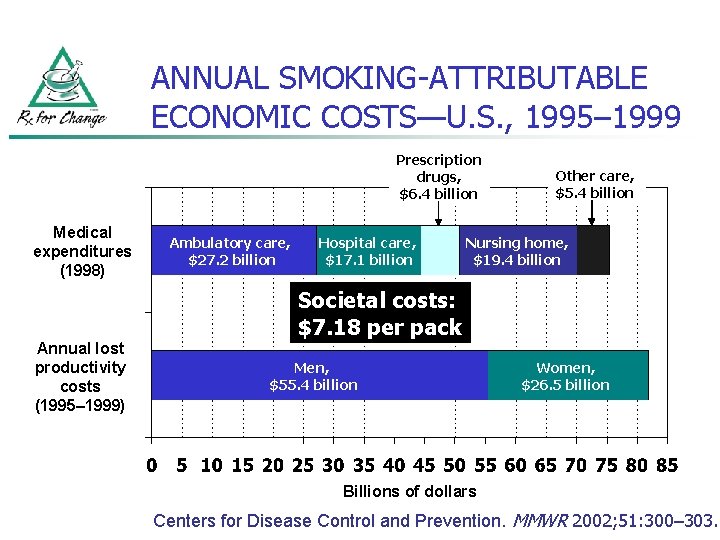
ANNUAL SMOKING-ATTRIBUTABLE ECONOMIC COSTS—U. S. , 1995– 1999 Prescription drugs, $6. 4 billion Medical expenditures (1998) Ambulatory care, $27. 2 billion Hospital care, $17. 1 billion Other care, $5. 4 billion Nursing home, $19. 4 billion Societal costs: $7. 18 per pack Annual lost productivity costs (1995– 1999) Men, $55. 4 billion Women, $26. 5 billion Billions of dollars Centers for Disease Control and Prevention. MMWR 2002; 51: 300– 303.

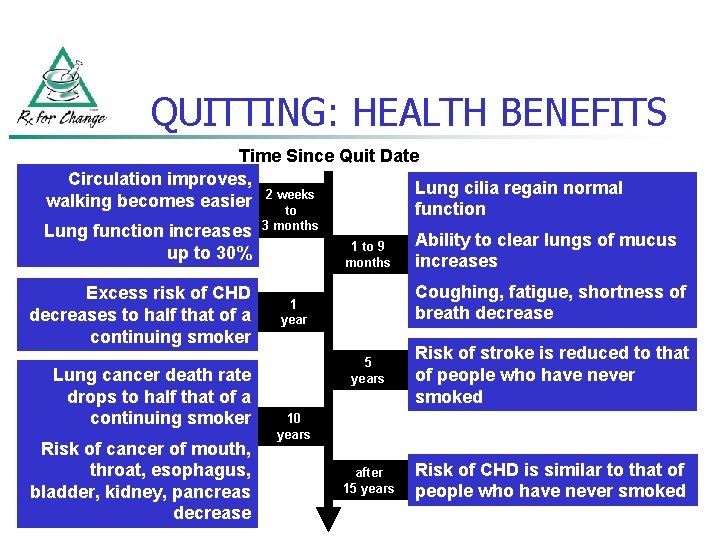
QUITTING: HEALTH BENEFITS Time Since Quit Date Circulation improves, Lung cilia regain normal walking becomes easier 2 weeks to function Lung function increases up to 30% Excess risk of CHD decreases to half that of a continuing smoker Lung cancer death rate drops to half that of a continuing smoker Risk of cancer of mouth, throat, esophagus, bladder, kidney, pancreas decrease 3 months 1 to 9 months Ability to clear lungs of mucus increases Coughing, fatigue, shortness of breath decrease 1 year 5 years Risk of stroke is reduced to that of people who have never smoked after 15 years Risk of CHD is similar to that of people who have never smoked 10 years

CLINICAL PRACTICE GUIDELINE for TREATING TOBACCO USE and DEPENDENCE n n Released June 2000 Sponsored by the AHRQ (Agency for Healthcare Research and Quality) of the USPHS (US Public Heath Service) with: n CDC (Centers for Disease Control) n NCI (National Cancer Institute) n NIDA (National Institute for Drug Addiction) n NHLBI (National Heart Lung & Blood Institute) n RWJF (Robert Wood Johnson Foundation) http: //www. surgeongeneral. gov/tobacco/

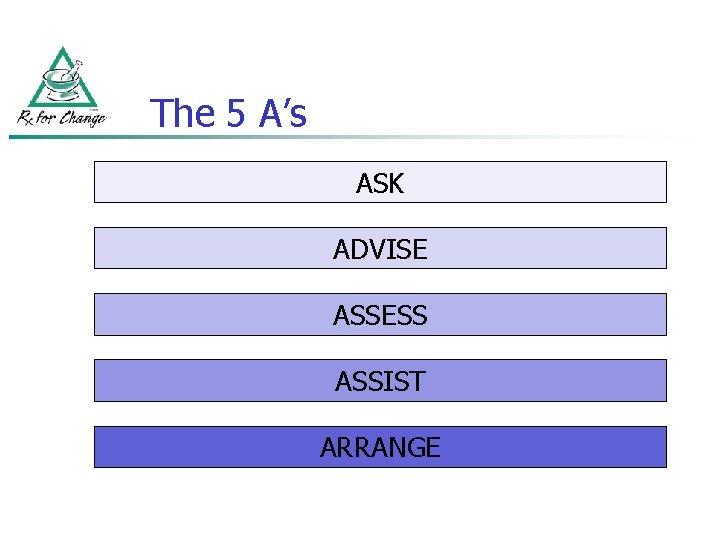
The 5 A’s ASK ADVISE ASSESS ASSIST ARRANGE

The 5 A’s n (cont’d) ASK about tobacco use Ask n “Do you ever smoke or use any type of tobacco? ” n “I take time to ask all of my patients about tobacco use—because it’s important. ”

The 5 A’s n (cont’d) ADVISE tobacco users to quit (clear, strong, personalized, sensitive) n n “It’s important that you quit as soon as possible, and I can help you. ” “I realize that quitting is difficult. It is the most important thing you can do to protect your health now and in the future. I have training to help my patients quit, and when you are ready, I will work with you to design a specialized treatment plan. ”

The 5 A’s (cont’d) n ASSESS readiness to make a quit attempt Assess n Assist ASSIST with the quit attempt

The 5 A’s n (cont’d) Arrange ARRANGE follow-up care Number of sessions Estimated quit rate* 0 to 1 12. 4% 2 to 3 16. 3% 4 to 8 More than 8 20. 9% 24. 7% * 5 months (or more) postcessation PROVIDE ASSISTANCE THROUGHOUT THE QUIT ATTEMPT Fiore et al. Treating Tobacco Use and Dependence. Clinical Practice Guideline. Rockville, MD: USDHHS, PHS, 2000.

THE 5 A’s: REVIEW ASK about tobacco USE ADVISE tobacco users to QUIT ASSESS readiness to make a QUIT attempt ASSIST with the QUIT ATTEMPT ARRANGE FOLLOW-UP care

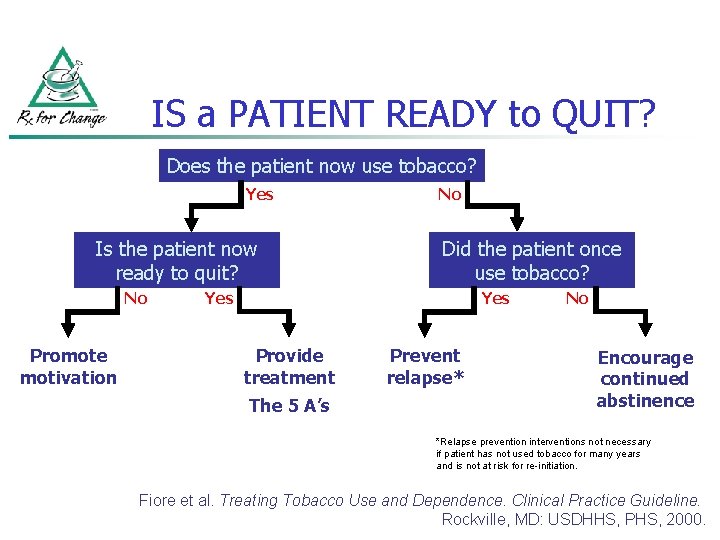
IS a PATIENT READY to QUIT? Does the patient now use tobacco? Yes Is the patient now ready to quit? No Promote motivation No Did the patient once use tobacco? Yes Provide treatment The 5 A’s Prevent relapse* No Encourage continued abstinence *Relapse prevention interventions not necessary if patient has not used tobacco for many years and is not at risk for re-initiation. Fiore et al. Treating Tobacco Use and Dependence. Clinical Practice Guideline. Rockville, MD: USDHHS, PHS, 2000.

PHARMACOTHERAPY “All patients attempting to quit should be encouraged to use effective pharmacotherapies for cessation except in the presence of special circumstances. ” Fiore et al. Treating Tobacco Use and Dependence. Clinical Practice Guideline. Rockville, MD: USDHHS, PHS, 2000.

PHARMACOLOGIC METHODS: FIRST-LINE THERAPIES Two general classes of FDA-approved drugs for cessation: 1. Nicotine replacement therapy § 2. Nicotine gum, patch, lozenge, nasal spray, inhaler Psychotropics § Sustained-release bupropion

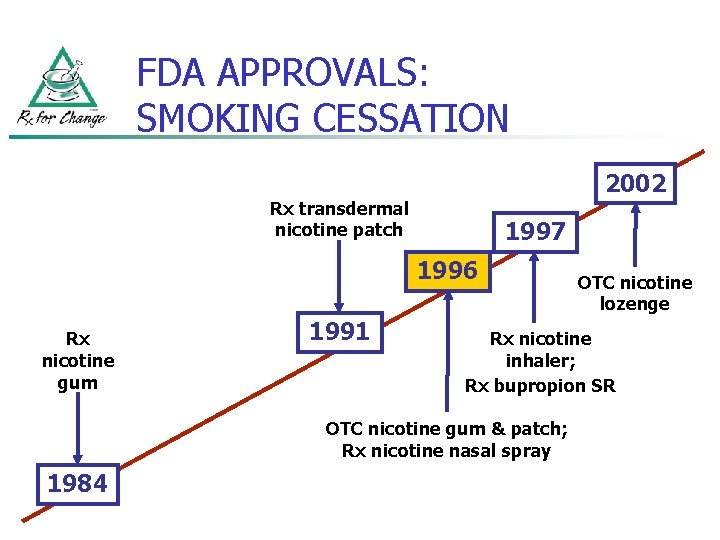
FDA APPROVALS: SMOKING CESSATION 2002 Rx transdermal nicotine patch 1997 1996 Rx nicotine gum 1991 Rx nicotine inhaler; Rx bupropion SR OTC nicotine gum & patch; Rx nicotine nasal spray 1984 OTC nicotine lozenge

NRT: RATIONALE for USE n n Reduces physical withdrawal from nicotine Allows patient to focus on behavioral and psychological aspects of tobacco cessation IMPROVES SUCCESS RATES

SYMPTOMS of NICOTINE WITHDRAWAL n Anger/irritability n Restlessness n Anxiety n Drowsiness n Cravings n Fatigue n Difficulty concentrating n n Hunger/weight gain Impaired task performance n Nervousness n Sleep disturbances n Impatience Hughes et al. Arch Gen Psychiatry 1991; 48: 52– 59.

NRT: PRODUCTS Polacrilex Gum n n Nicorette (OTC) Generic nicotine gum (OTC) Lozenge n Commit (OTC) Transdermal Patches n n n Nicoderm CQ (OTC) Nicotrol (OTC) Generic nicotine patches (OTC, Rx) Nasal Spray n Nicotrol NS (Rx) Inhaler n Nicotrol (Rx)

PLASMA NICOTINE CONCENTRATIONS for NICOTINE-CONTAINING PRODUCTS Cigarette Moist snuff 0 10 20 30 Time (minutes) 40 50 60

NRT: PRECAUTIONS n Patients with underlying cardiovascular disease n Recent myocardial infarction n Life-threatening arrhythmias n Severe or worsening angina

NRT: PRECAUTIONS n (cont’d) Patients with other underlying conditions n Active temporomandibular joint disease (gum only) n Pregnancy n Lactation Minimum age for FDA-approved NRT use: 18 years

NICOTINE GUM: Nicorette; generic (Glaxo. Smith. Kline; Watson Labs) n Approved for Rx use in 1984; OTC in 1996 n Resin complex n n n Nicotine Polacrilin Sugar-free chewing gum base Buffering agents to enhance buccal absorption of nicotine Available: 2 mg, 4 mg; regular, mint, orange

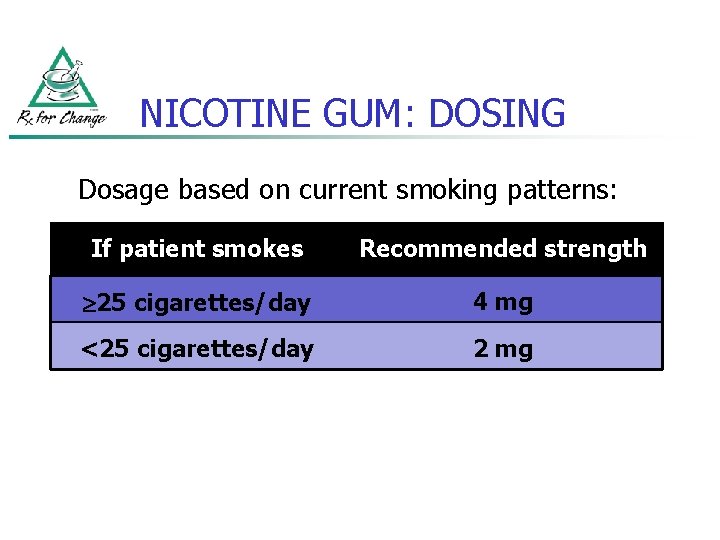
NICOTINE GUM: DOSING Dosage based on current smoking patterns: If patient smokes Recommended strength 25 cigarettes/day 4 mg <25 cigarettes/day 2 mg

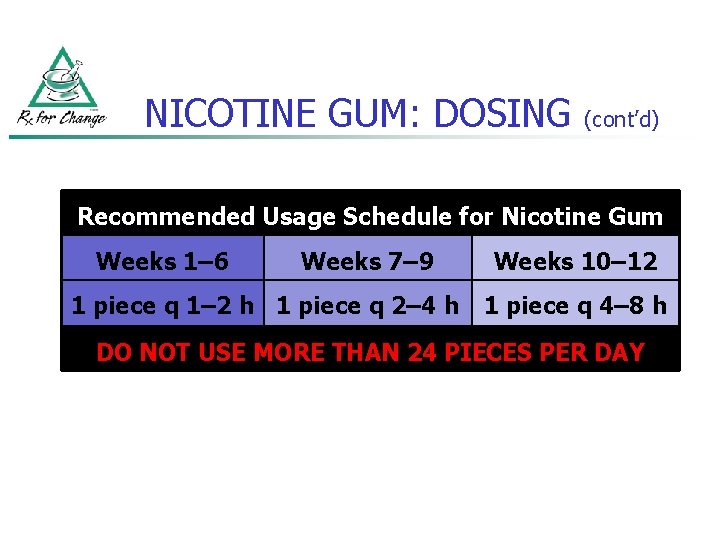
NICOTINE GUM: DOSING (cont’d) Recommended Usage Schedule for Nicotine Gum Weeks 1– 6 Weeks 7– 9 1 piece q 1– 2 h 1 piece q 2– 4 h Weeks 10– 12 1 piece q 4– 8 h DO NOT USE MORE THAN 24 PIECES PER DAY

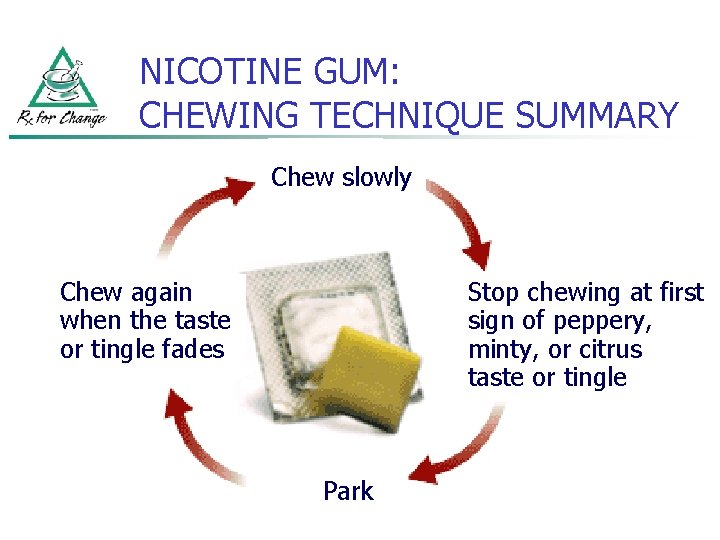
NICOTINE GUM: DIRECTIONS for USE n n Use gum according to recommended dosing schedule (to decrease cravings & withdrawal symptoms) Chew each piece very slowly several times Stop chewing at first sign of peppery, minty, or citrus taste or of slight tingling in mouth (~15 chews, but varies) “Park” gum between cheek & gum (to allow absorption of nicotine across buccal mucosa)

NICOTINE GUM: DIRECTIONS for USE n n n (cont’d) Resume slow chewing when taste or tingle fades When taste or tingle returns, stop and park gum in different place in mouth Repeat chew/park steps until most of the nicotine is gone (taste or tingle does not return; generally 30 minutes)

NICOTINE GUM: CHEWING TECHNIQUE SUMMARY Chew slowly Chew again when the taste or tingle fades Stop chewing at first sign of peppery, minty, or citrus taste or tingle Park

NICOTINE GUM: GRADUAL REDUCTION of DOSE Recommended strategies for discontinuing use of nicotine gum: n n n Chew gum for 10– 15 minutes instead of 30 minutes Chew each piece for more than 30 minutes but reduce the number of pieces used daily Substitute ordinary chewing gum for nicotine gum

NICOTINE GUM: ADDITIONAL PATIENT EDUCATION n n To improve chances of quitting, use at least nine pieces of gum daily The effectiveness of nicotine gum may be reduced by some foods and beverages: Coffee Juices Wine Soft drinks Do NOT eat or drink for 15 minutes BEFORE or while using nicotine gum.

NICOTINE GUM: ADD’L PATIENT EDUCATION n n (cont’d) Chewing gum will not provide same rapid satisfaction that smoking provides Chewing gum too rapidly can cause excessive release of nicotine, resulting in n Lightheadedness n Nausea/vomiting n Irritation of throat and mouth n Hiccups n Indigestion

NICOTINE GUM: ADD’L PATIENT EDUCATION n n (cont’d) Side effects of nicotine gum include n Mouth soreness n Hiccups n Dyspepsia n Jaw muscle ache Nicotine gum may stick to dental work n Discontinue use if excessive sticking or damage to dental work occurs

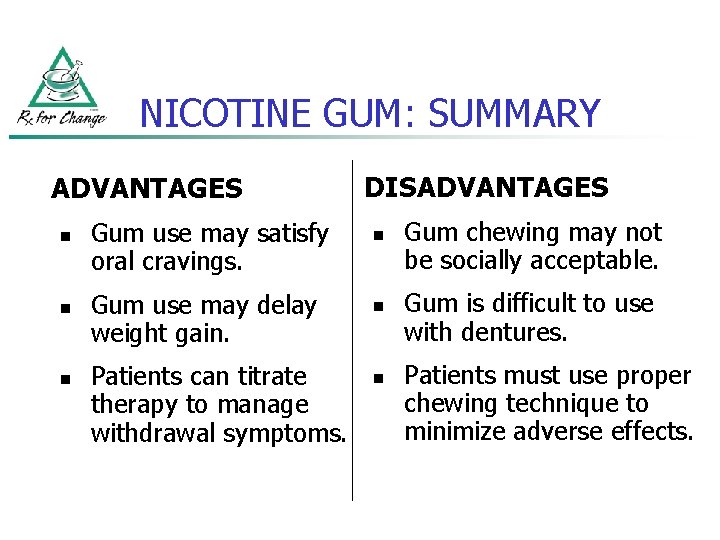
NICOTINE GUM: SUMMARY ADVANTAGES n n n Gum use may satisfy oral cravings. Gum use may delay weight gain. Patients can titrate therapy to manage withdrawal symptoms. DISADVANTAGES n n n Gum chewing may not be socially acceptable. Gum is difficult to use with dentures. Patients must use proper chewing technique to minimize adverse effects.

NICOTINE LOZENGE Commit (Glaxo. Smith. Kline) n n Approved for OTC use in 2002 Nicotine polacrilex formulation n n Delivers ~25% more nicotine than equivalent gum dose Available: 2 mg, 4 mg

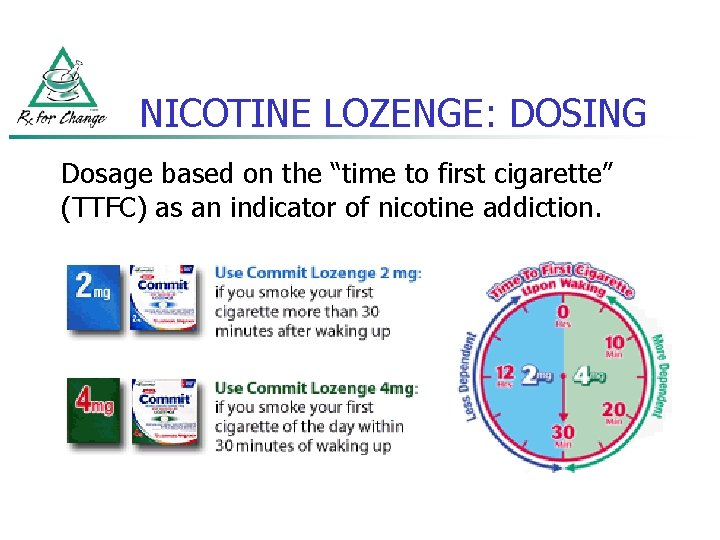
NICOTINE LOZENGE: DOSING Dosage based on the “time to first cigarette” (TTFC) as an indicator of nicotine addiction.

NICOTINE LOZENGE: DOSING (cont’d) Recommended Usage Schedule for Commit Lozenge Weeks 1– 6 Weeks 7– 9 Weeks 10– 12 1 lozenge q 1– 2 h q 2– 4 h q 4– 8 h DO NOT USE MORE THAN 20 LOZENGES PER DAY

NICOTINE LOZENGE: DIRECTIONS for USE (cont’d) n n n Do not chew or swallow the lozenge Occasionally rotate the lozenge to different areas of the mouth Lozenge will completely dissolve in about 20 30 minutes

NICOTINE LOZENGE: ADDITIONAL PATIENT EDUCATION n n n To improve chances of quitting, use at least nine lozenges daily during the first 6 weeks The lozenge will not provide same rapid satisfaction that smoking provides The effectiveness of nicotine lozenge may be reduced by some foods and beverages: Coffee Wine Juices Soft drinks Do NOT eat or drink for 15 minutes BEFORE or while using nicotine lozenge.

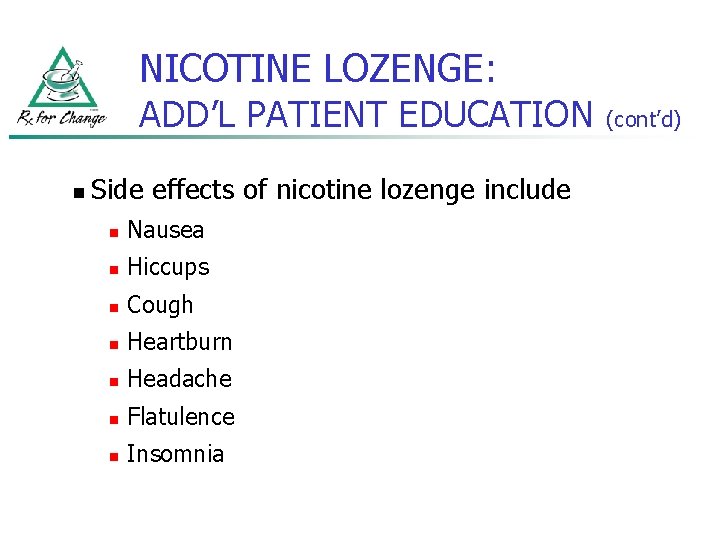
NICOTINE LOZENGE: ADD’L PATIENT EDUCATION n Side effects of nicotine lozenge include n Nausea n Hiccups n Cough n Heartburn n Headache n Flatulence n Insomnia (cont’d)

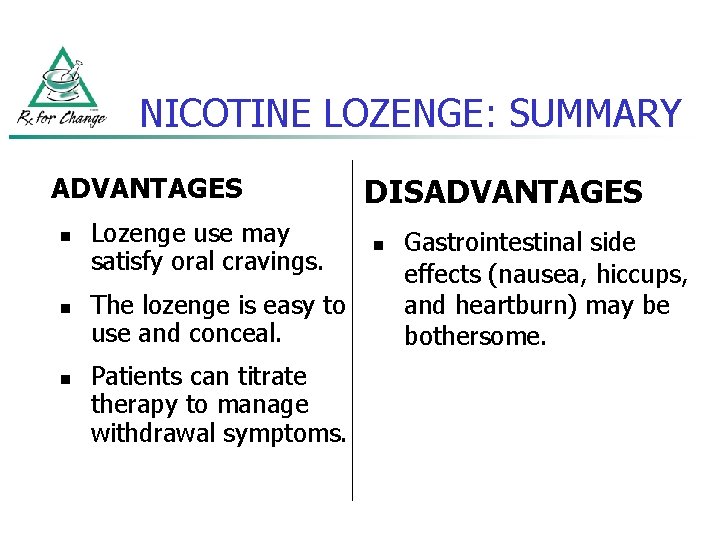
NICOTINE LOZENGE: SUMMARY ADVANTAGES n n n Lozenge use may satisfy oral cravings. The lozenge is easy to use and conceal. Patients can titrate therapy to manage withdrawal symptoms. DISADVANTAGES n Gastrointestinal side effects (nausea, hiccups, and heartburn) may be bothersome.

TRANSDERMAL NICOTINE PATCH n Approved for Rx use in 1991; OTC in 1996 n Current products include n n n Nicoderm CQ Patch. OTC (Glaxo. Smith. Kline) Nicotrol Patch. OTC (Pharmacia) Generic Products. Rx, OTC

TRANSDERMAL NICOTINE PATCH n n n Nicotine is well absorbed across the skin Delivery to systemic circulation avoids hepatic first-pass metabolism Plasma nicotine levels are lower, fluctuate less than with smoking n n Relieve nicotine withdrawal Low potential for dependence (compared to rapid delivery systems)

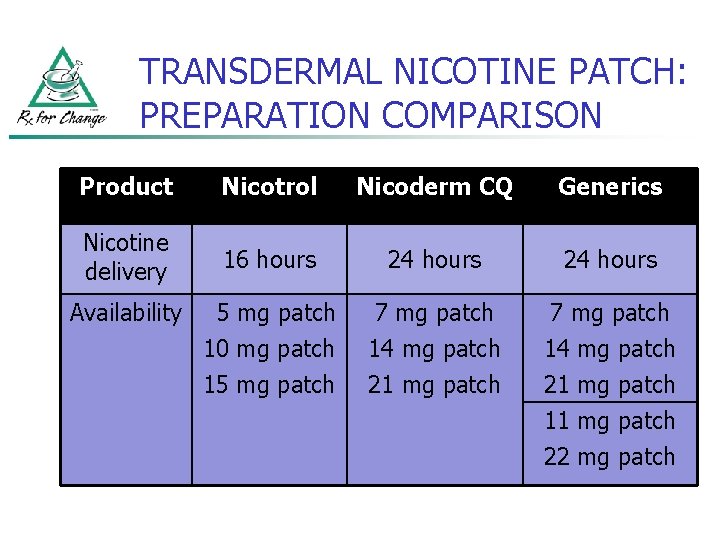
TRANSDERMAL NICOTINE PATCH: PREPARATION COMPARISON Product Nicotrol Nicoderm CQ Generics Nicotine delivery 16 hours 24 hours 5 mg patch 10 mg patch 15 mg patch 7 mg patch 14 mg patch 21 mg patch Availability 11 mg patch 22 mg patch

TRANSDERMAL NICOTINE PATCH: DOSING Product Light Smoker Heavy Smoker 10 cigarettes/day Not indicated >10 cigarettes/day Step 1 (15 mg x 6 weeks) Step 2 (10 mg x 2 weeks) Step 3 (5 mg x 2 weeks) 10 cigarettes/day Step 2 (14 mg x 6 weeks) Step 3 (7 mg x 2 weeks) >10 cigarettes/day Step 1 (21 mg x 6 weeks) Step 2 (14 mg x 2 weeks) Step 3 (7 mg x 2 weeks) 10 cigarettes/day Generic (formerly Habitrol) Step 2 (14 mg x 6 weeks) Step 3 (7 mg x 2 weeks) >10 cigarettes/day Step 1 (21 mg x 4 weeks) Step 2 (14 mg x 2 weeks) Step 3 (7 mg x 2 weeks) 15 cigarettes/day Generic (formerly Pro. Step) 11 mg x 6 weeks >15 cigarettes/day 22 mg x 6 weeks Nicotrol Nicoderm CQ

TRANSDERMAL NICOTINE PATCH: DIRECTIONS for USE n n Choose an area of skin on the upper body or the upper outer part of the arm Make sure the skin is clean, dry, and hairless Hair will interfere with application of the patch Do not shave; this may irritate the skin

TRANSDERMAL NICOTINE PATCH: DIRECTIONS for USE (cont’d) n n n Do not apply patch to skin that is inflamed, burned, or irritated in any way (these conditions may alter nicotine absorption) Apply patch to a different area each day The same area should not be used again for at least 1 week

TRANSDERMAL NICOTINE PATCH: DIRECTIONS for USE (cont’d) n Remove patch from protective pouch

TRANSDERMAL NICOTINE PATCH: DIRECTIONS for USE (cont’d) n Peel off half of the backing from the patch

TRANSDERMAL NICOTINE PATCH: DIRECTIONS for USE (cont’d) n n Apply adhesive side of patch to the skin Peel off remaining protective covering Press firmly with palm of hand for 10 seconds Make sure the patch sticks well to skin, especially around the edges

TRANSDERMAL NICOTINE PATCH: DIRECTIONS for USE (cont’d) n n Wash hands (nicotine on hands can get into eyes or nose and cause stinging or redness) Do not leave the patch on skin for more than 16 hours (Nicotrol) or 24 hours (Nicoderm, generic patches—doing so may lead to skin irritation Adhesive remaining on skin may be removed with rubbing alcohol or acetone Dispose of a used patch by folding onto itself, completely covering the adhesive area

TRANSDERMAL NICOTINE PATCH: ADDITIONAL PATIENT EDUCATION n n n Water will not harm the nicotine patch if it is applied correctly; patients may bathe, swim, shower, or exercise while wearing the patch Do not cut patches to adjust dose n Nicotine will evaporate rapidly n Patch will be rendered useless Keep new and used patches out of the reach of children and pets

TRANSDERMAL NICOTINE PATCH: ADD’L PATIENT EDUCATION (cont’d) n Side effects to expect in first hour: n n n Mild itching Burning Tingling After patch removal, the skin may appear red for the next 24 hours If skin stays red more than 4 days or swells, or if a rash appears, contact health care provider; do not put on a new patch

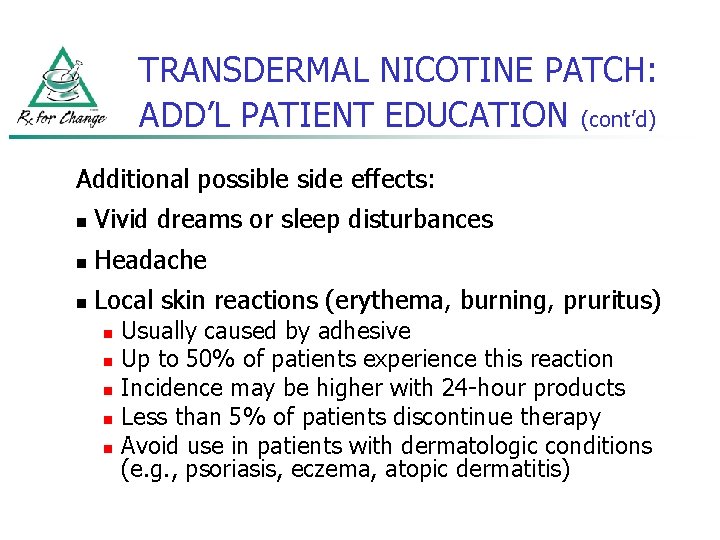
TRANSDERMAL NICOTINE PATCH: ADD’L PATIENT EDUCATION (cont’d) Additional possible side effects: n Vivid dreams or sleep disturbances n Headache n Local skin reactions (erythema, burning, pruritus) n n n Usually caused by adhesive Up to 50% of patients experience this reaction Incidence may be higher with 24 -hour products Less than 5% of patients discontinue therapy Avoid use in patients with dermatologic conditions (e. g. , psoriasis, eczema, atopic dermatitis)

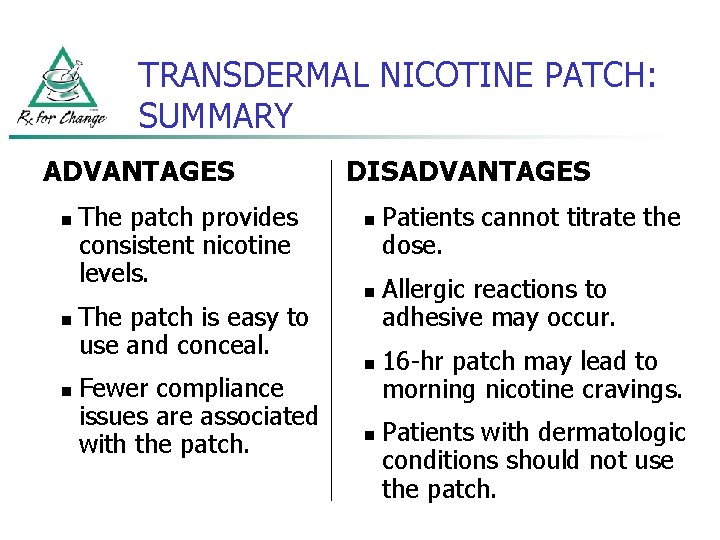
TRANSDERMAL NICOTINE PATCH: SUMMARY ADVANTAGES n n n The patch provides consistent nicotine levels. The patch is easy to use and conceal. Fewer compliance issues are associated with the patch. DISADVANTAGES n n Patients cannot titrate the dose. Allergic reactions to adhesive may occur. 16 -hr patch may lead to morning nicotine cravings. Patients with dermatologic conditions should not use the patch.

LONG-TERM ( 6 month) QUIT RATES for AVAILABLE CESSATION MEDICATIONS Percent quit 23. 9 19. 7 19. 3 17. 2 17. 1 14. 4 11. 8 11. 5 8. 4 8. 9 9. 1 10. 2 Data adapted from Silagy et al. Cochrane Database Syst Rev, 2002 and Hughes et al. , Cochrane Database Syst Rev, 2000

COMBINATION NRT Long-acting formulation (patch) n Produces relatively constant levels of nicotine PLUS Short-acting formulation (gum, lozenge, inhaler, nasal spray) n Allows for acute dose titration as needed for withdrawal symptoms Reserve for patients unable to quit using monotherapy.

COMPARATIVE DAILY COSTS of PHARMACOTHERAPY $6. 07 $5. 81 $4. 98 $2. 79 in KY $4. 81 in NJ $4. 30 $3. 91 $3. 40 Cost per day, in U. S. dollars

The RESPONSIBILITY of HEALTH PROFESSIONALS It is inconsistent to provide health care and —at the same time— remain silent (or inactive) about a major health risk. TOBACCO CESSATION is an important component of THERAPY.

DR. GRO HARLEM BRUNTLAND, DIRECTOR-GENERAL of the WHO: “If we do not act decisively, a hundred years from now our grandchildren and their children will look back and seriously question how people claiming to be committed to public health and social justice allowed the tobacco epidemic to unfold unchecked. ” US Department of Health and Human Services. Women and Smoking: A Report of the Surgeon General. Washington, DC: Public Health Service, 2001.
 18004149025
18004149025 Conclusion of cigarette smoking
Conclusion of cigarette smoking Example of combustion reaction
Example of combustion reaction Occupational therapy intervention plan for hip arthroplasty
Occupational therapy intervention plan for hip arthroplasty Rrt aeiou
Rrt aeiou Electrolyte replacement therapy
Electrolyte replacement therapy Nicotine delivery
Nicotine delivery What does the wine symbolize in persepolis
What does the wine symbolize in persepolis Nicotine
Nicotine Carcinogens in cigarette smoke
Carcinogens in cigarette smoke Cigarette warning label
Cigarette warning label Cigarette radiation sievert
Cigarette radiation sievert Cigarette 365 ats
Cigarette 365 ats Nicotine verslaving
Nicotine verslaving Belladonna tomato
Belladonna tomato Daily cigarette consumption
Daily cigarette consumption What nicotine looks like
What nicotine looks like Carcinogens in cigarette smoke
Carcinogens in cigarette smoke Nicotine acetylcholine receptors
Nicotine acetylcholine receptors Nicotine excretion
Nicotine excretion Nicotine patch dosing chart
Nicotine patch dosing chart New nicotine alliance
New nicotine alliance Every time jarrad opens his cigarette case
Every time jarrad opens his cigarette case Nicotine history
Nicotine history Nicotine withdrawal
Nicotine withdrawal Nicotine acetylcholine receptors
Nicotine acetylcholine receptors Nicotine delivery
Nicotine delivery Publicidad subliminal camel
Publicidad subliminal camel Nicotine+
Nicotine+ Euromonitor international
Euromonitor international Black fly in your chardonnay
Black fly in your chardonnay Nicotine p listed waste
Nicotine p listed waste Nicotine
Nicotine Humanistic therapies aim to boost
Humanistic therapies aim to boost Psychoanalytic therapy is to as humanistic therapy is to
Psychoanalytic therapy is to as humanistic therapy is to Bioness bits cost
Bioness bits cost Logical fallacies in thank you for smoking
Logical fallacies in thank you for smoking Pasive smoking
Pasive smoking Smoking cessation learning objectives
Smoking cessation learning objectives Characteristics of a paragraph
Characteristics of a paragraph Quentin staples
Quentin staples Melissa moore smoking
Melissa moore smoking Chatzy smoking
Chatzy smoking Personal pronouns in persuasive writing
Personal pronouns in persuasive writing Being sold
Being sold Smoking history taking
Smoking history taking Fiona stopped smoking last year
Fiona stopped smoking last year Conclusion of smoking
Conclusion of smoking 니코틴성 구내염
니코틴성 구내염 Creating supportive environments smoking
Creating supportive environments smoking Dying bryan
Dying bryan Tobacco true or false worksheet
Tobacco true or false worksheet Smokers radiation
Smokers radiation Would you mind not talking during the lesson
Would you mind not talking during the lesson Type of smoking
Type of smoking Kathleen quinlan smoking
Kathleen quinlan smoking 30 pack year smoking history means
30 pack year smoking history means Smoking cessation learning objectives
Smoking cessation learning objectives Smoking bamboo treatment
Smoking bamboo treatment Smoking
Smoking Stemi lead
Stemi lead How the nervous system works
How the nervous system works 5 as smoking cessation flow chart
5 as smoking cessation flow chart Pack years
Pack years Who are second hand smokers
Who are second hand smokers Poland
Poland