Nitrous Oxide N 2 O and Stratospheric Ozone









- Slides: 26

Nitrous Oxide (N 2 O) and Stratospheric Ozone Layer Depletion A. R. Ravishankara Earth System Research Laboratory, Chemical Sciences Division, National Oceanic and Atmospheric Administration, Boulder, CO, USA 1. Nitrous Oxide (N 2 O): The Dominant Ozone-Depleting Substance Emitted in the 21 st Century, A. R. Ravishankara, John S. Daniel, and Robert W. Portmann, SCIENCE, Vol. 326, Pages: 123 -125, 2009 (OCT 2) 2. Options to accelerate ozone recovery: ozone and climate benefits, J. S. Daniel, E. L. Fleming, G. J. M. Velders, C. H. Jackman, and A. R. Ravishankara, Atoms. Chem. Phys. , 10, 76977707, 2010. 3. Ozone depletion due to N 2 O: influences of other gases, R. W. Portmann, J. S. Daniel, A. R. Ravishankara, Phil. Trans. . Royal Soc. , B (Biology), The proceedings of the discussion meeting entitled ‘Nitrous oxide, the forgotten greenhouse gas’, held between 23 -24 May, 2011 at The Royal Society Kavli Centre. In press, 2011/2012. Disclaimer: I am not talking as a co-Chair of WMO/ENEP SAP panel Opinions expressed are mine and not necessarily those of NOAA

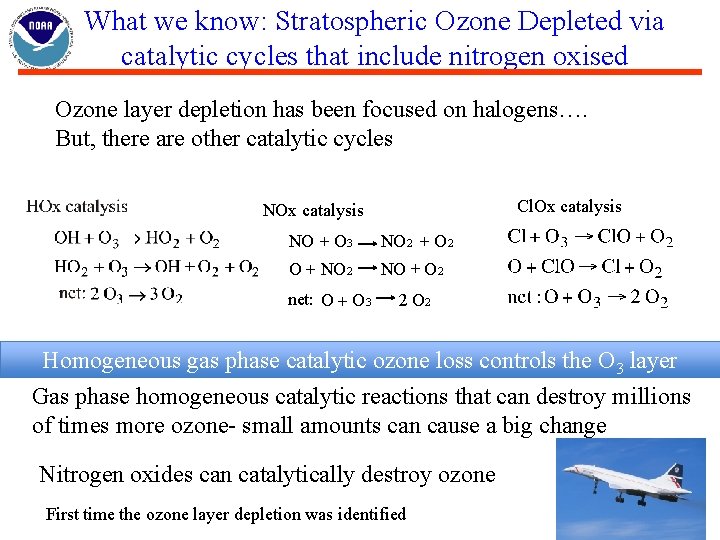
What we know: Stratospheric Ozone Depleted via catalytic cycles that include nitrogen oxised Ozone layer depletion has been focused on halogens…. But, there are other catalytic cycles Cl. Ox catalysis NO + O 3 O + NO 2 net: O + O 3 NO 2 + O 2 NO + O 2 2 O 2 Homogeneous gas phase catalytic ozone loss controls the O 3 layer Gas phase homogeneous catalytic reactions that can destroy millions of times more ozone- small amounts can cause a big change Nitrogen oxides can catalytically destroy ozone First time the ozone layer depletion was identified

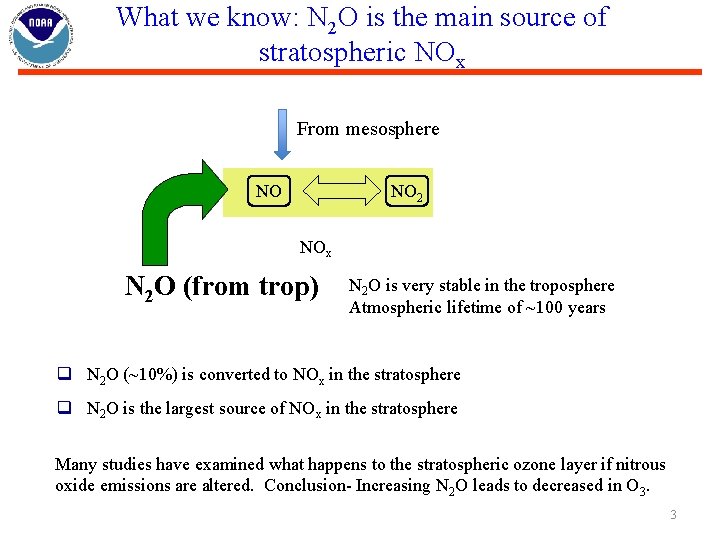
What we know: N 2 O is the main source of stratospheric NOx From mesosphere NO NO 2 NOx N 2 O (from trop) N 2 O is very stable in the troposphere Atmospheric lifetime of ~100 years q N 2 O (~10%) is converted to NOx in the stratosphere q N 2 O is the largest source of NOx in the stratosphere Many studies have examined what happens to the stratospheric ozone layer if nitrous oxide emissions are altered. Conclusion- Increasing N 2 O leads to decreased in O 3. 3

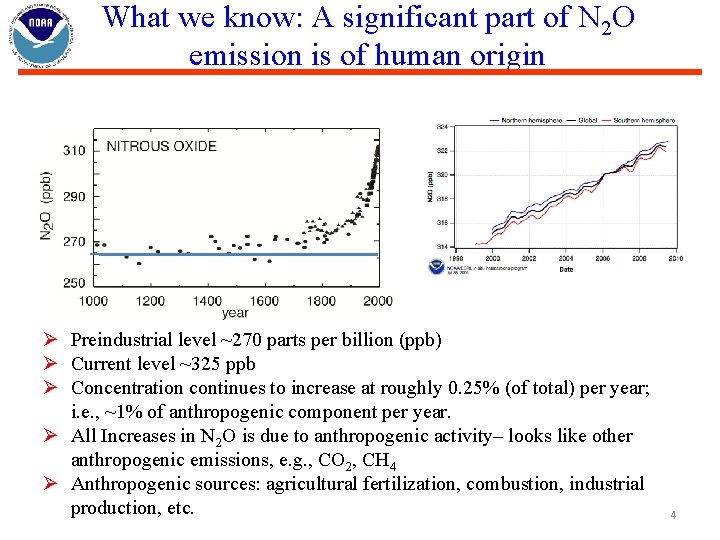
What we know: A significant part of N 2 O emission is of human origin Ø Preindustrial level ~270 parts per billion (ppb) Ø Current level ~325 ppb Ø Concentration continues to increase at roughly 0. 25% (of total) per year; i. e. , ~1% of anthropogenic component per year. Ø All Increases in N 2 O is due to anthropogenic activity– looks like other anthropogenic emissions, e. g. , CO 2, CH 4 Ø Anthropogenic sources: agricultural fertilization, combustion, industrial production, etc. 4

What we know: Others have studied the effect of N 2 O increases on ozone layer Many studies have examined what happens to the stratospheric ozone layer if nitrous oxide emissions are altered Examples: o. Kinnison et al. (1988) o. Randeniya et al. (2002) o. Chipperfield and Feng (2003) o. Konopka et al. (2007) oetc. All indicate that ozone would decrease if N 2 O increases, as expected from emission trends 5

What we did View anthropogenic N 2 O as a potential ozone depleting substance - the same way as the ozone depleting substances (ODSs) controlled by the Montreal Protocol 6

Ozone Depletion Potential of N 2 O Calculated ODP using Garcia-Solomon 2 D model for 2000 conditions ODP of N 2 O = 0. 017 N 2 O is an ozone-depleting gas! This positive number for ODP is comparable to those of some of the HCFCs: HCFC-123 = 0. 02; HCFC-124 = 0. 022; HCFC-225 ca = 0. 025; HCFC-225 cb = 0. 033 To our knowledge, this is the first time N 2 O is suggested to be an ozone-depleting substance in the same way that other Montreal Protocol gases 7

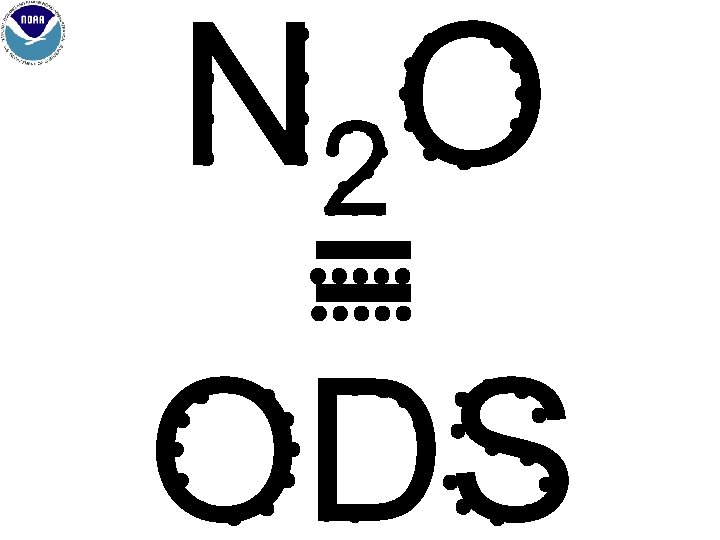
N 2 O = ODS

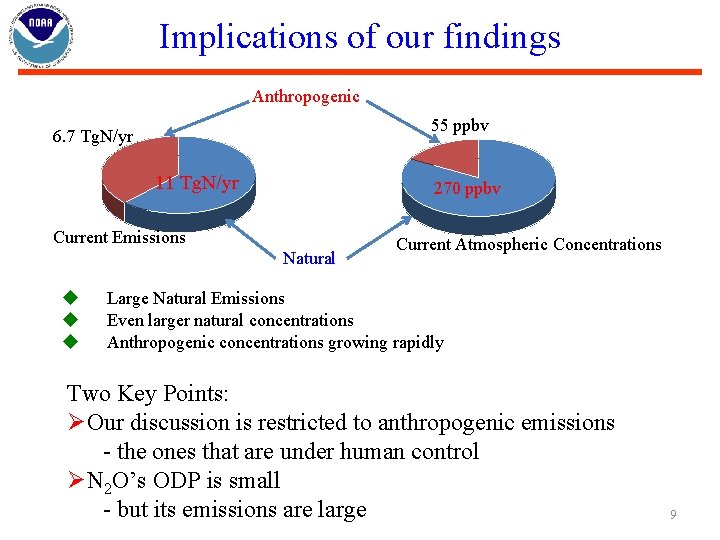
Implications of our findings Anthropogenic 55 ppbv 6. 7 Tg. N/yr 11 Tg. N/yr 1 2 Current Emissions Natural u u u 270 ppbv 1 2 Current Atmospheric Concentrations Large Natural Emissions Even larger natural concentrations Anthropogenic concentrations growing rapidly Two Key Points: ØOur discussion is restricted to anthropogenic emissions - the ones that are under human control ØN 2 O’s ODP is small - but its emissions are large 9

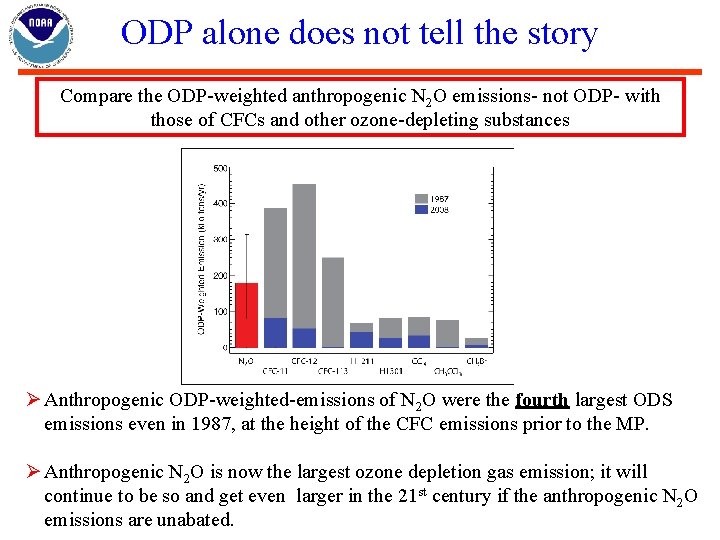
ODP alone does not tell the story Compare the ODP-weighted anthropogenic N 2 O emissions- not ODP- with those of CFCs and other ozone-depleting substances Ø Anthropogenic ODP-weighted-emissions of N 2 O were the fourth largest ODS emissions even in 1987, at the height of the CFC emissions prior to the MP. Ø Anthropogenic N 2 O is now the largest ozone depletion gas emission; it will continue to be so and get even larger in the 21 st century if the anthropogenic N 2 O emissions are unabated.

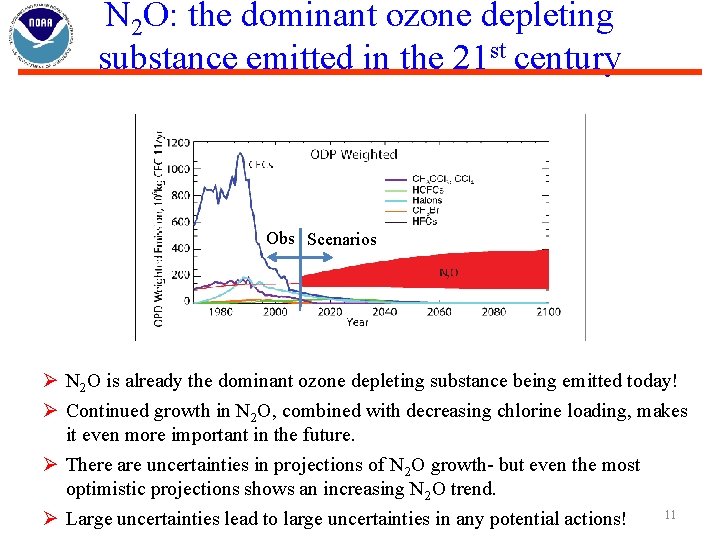
N 2 O: the dominant ozone depleting substance emitted in the 21 st century Obs Scenarios Ø N 2 O is already the dominant ozone depleting substance being emitted today! Ø Continued growth in N 2 O, combined with decreasing chlorine loading, makes it even more important in the future. Ø There are uncertainties in projections of N 2 O growth- but even the most optimistic projections shows an increasing N 2 O trend. 11 Ø Large uncertainties lead to large uncertainties in any potential actions!

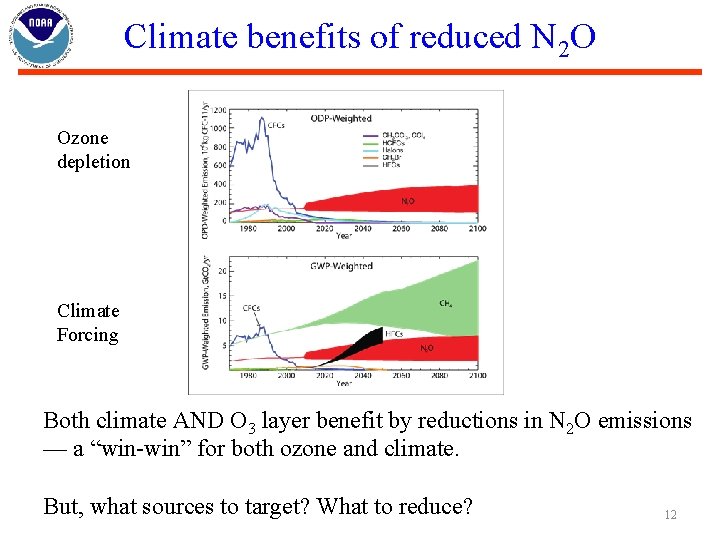
Climate benefits of reduced N 2 O Ozone depletion Climate Forcing Both climate AND O 3 layer benefit by reductions in N 2 O emissions — a “win-win” for both ozone and climate. But, what sources to target? What to reduce? 12

A few other points of note Ø N 2 O does not contribute to the Antarctic ozone hole. It influences global ozone Ø Changes in anthropogenic N 2 O emissions will affect the estimated date for the recovery of the global ozone layer Ø Calls in to question the “baseline” for ozone recovery Ø Anthropogenic N 2 O could be an unintended byproduct of climate mitigation strategies, e. g. , biofuel, iron fertilization Ø Ozone depletion by anthropogenic N 2 O is roughly the same as that from the original projections for 500 US supersonic transport SSTs. 13

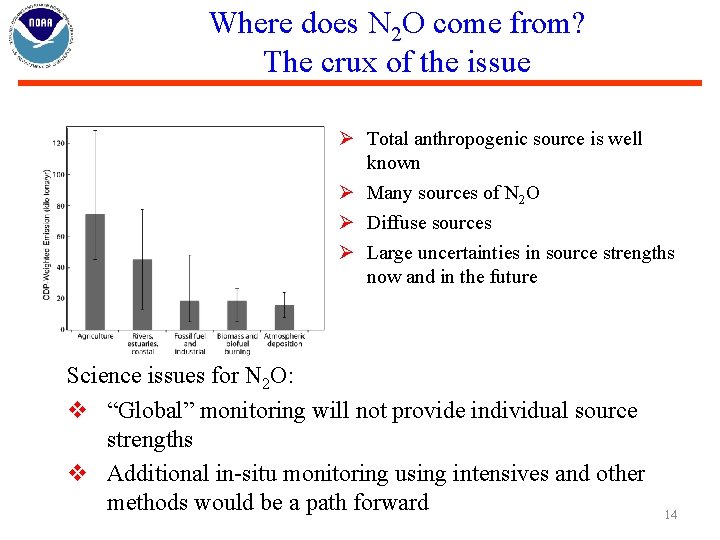
Where does N 2 O come from? The crux of the issue Ø Total anthropogenic source is well known Ø Many sources of N 2 O Ø Diffuse sources Ø Large uncertainties in source strengths now and in the future Science issues for N 2 O: v “Global” monitoring will not provide individual source strengths v Additional in-situ monitoring using intensives and other methods would be a path forward 14

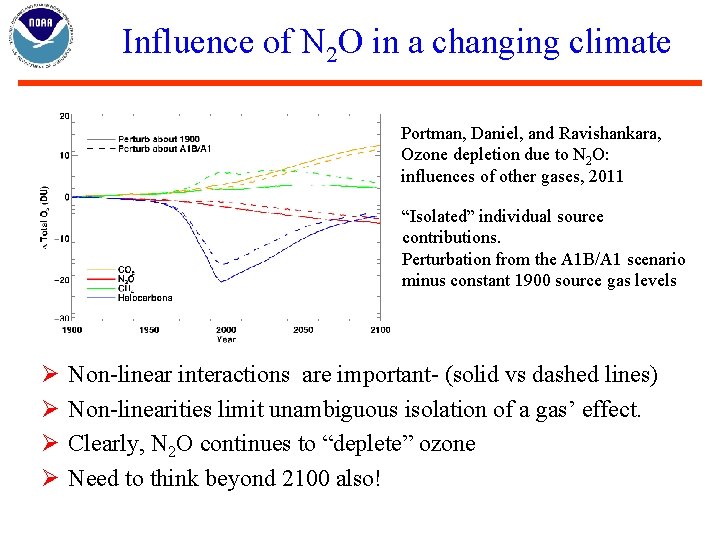
Influence of N 2 O in a changing climate Portman, Daniel, and Ravishankara, Ozone depletion due to N 2 O: influences of other gases, 2011 “Isolated” individual source contributions. Perturbation from the A 1 B/A 1 scenario minus constant 1900 source gas levels Ø Ø Non-linear interactions are important- (solid vs dashed lines) Non-linearities limit unambiguous isolation of a gas’ effect. Clearly, N 2 O continues to “deplete” ozone Need to think beyond 2100 also!

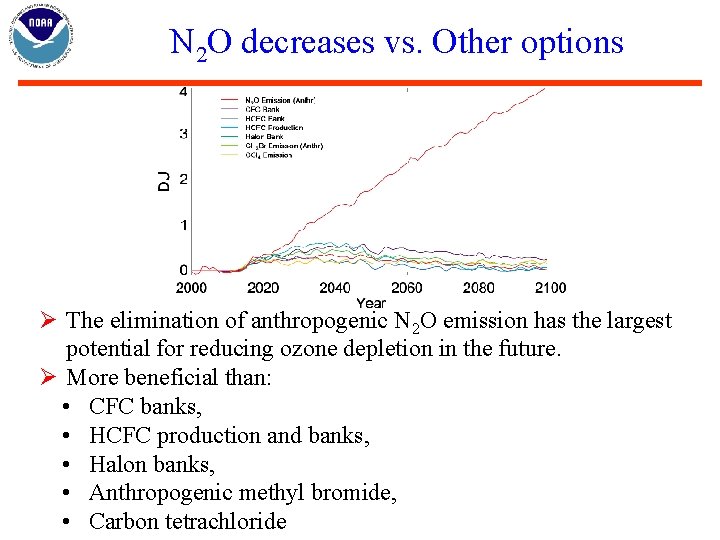
N 2 O decreases vs. Other options Ø The elimination of anthropogenic N 2 O emission has the largest potential for reducing ozone depletion in the future. Ø More beneficial than: • CFC banks, • HCFC production and banks, • Halon banks, • Anthropogenic methyl bromide, • Carbon tetrachloride

Key Points of Our Studies 1. Fact: NOx from N 2 O leads to ozone depletion; N 2 O is not regulated under the Montreal Protocol. 2. Treated N 2 O like any other ozone-depleting substance (CFCs, Halons, methyl bromide, …). Calculated ODP. Compared ODPweighted emissions of anthropogenic N 2 O to the ODP-weighted anthropogenic emissions of other ozone-depleting substances. 3. Looked at the influence of “climate change” on N 2 O’s ozone depletion. Compared Magnitudes of anthropogenic N 2 O-induced depletion with other standard measures- e. g. , destruction of banks 4. Findings: Anthropogenic N 2 O is now the largest manmade ozonedepleting gas emission (a recent development owing to the successful abatement of CFCs and other ODSs under the Montreal Protocol!), and it will remain so for the next century if anthropogenic 17 emissions of N 2 O are unabated.

Thank you for your attention

Backup slides

What we know: Current state and outlook based on assessments Ozone layer depletion has been focused on halogens…. Findings from 2002 and 2006 SAP of UNEP/WMO: Ø The Montreal Protocol is a success! Ø The chlorine (and bromine) containing ODSs are decreasing in the atmosphere Ø The ozone layer is expected to recover Ø It is showing signs of recovery Ø Climate change and atmospheric composition will influence the recovery 20

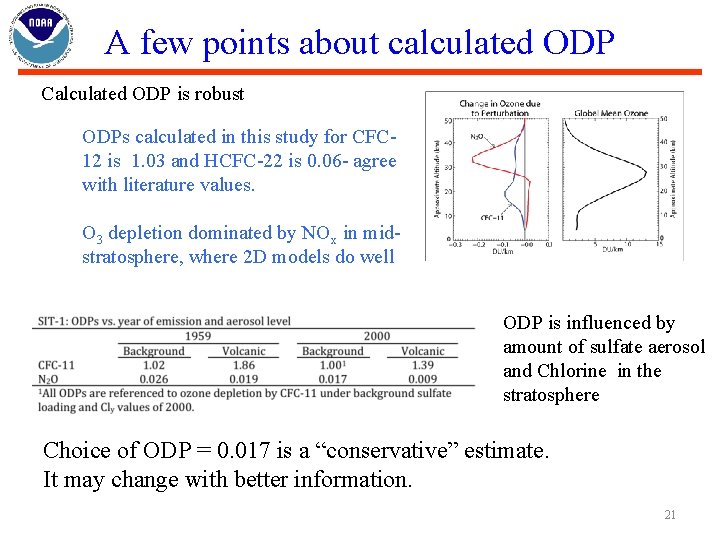
A few points about calculated ODP Calculated ODP is robust ODPs calculated in this study for CFC 12 is 1. 03 and HCFC-22 is 0. 06 - agree with literature values. O 3 depletion dominated by NOx in midstratosphere, where 2 D models do well ODP is influenced by amount of sulfate aerosol and Chlorine in the stratosphere Choice of ODP = 0. 017 is a “conservative” estimate. It may change with better information. 21

Why is the ODP of N 2 O ~0. 02? NOx catalysis is roughly similar in efficiency to that by Cl. OX Main reason for smaller value: Only 10% of N 2 O is converted to NOx and while 3 Cl atoms are produced from CFC-11 (CFCl 3) There are some differences in efficiency, fraction in active form, etc. that account for the other difference 22

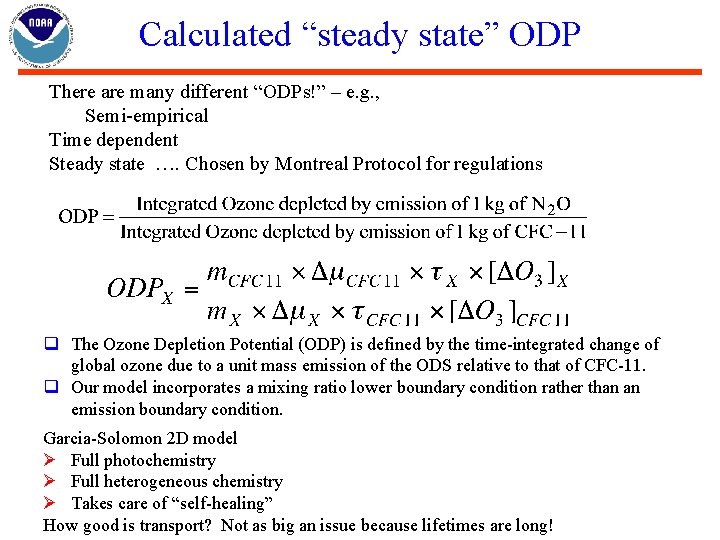
Calculated “steady state” ODP There are many different “ODPs!” – e. g. , Semi-empirical Time dependent Steady state …. Chosen by Montreal Protocol for regulations q The Ozone Depletion Potential (ODP) is defined by the time-integrated change of global ozone due to a unit mass emission of the ODS relative to that of CFC-11. q Our model incorporates a mixing ratio lower boundary condition rather than an emission boundary condition. Garcia-Solomon 2 D model Ø Full photochemistry Ø Full heterogeneous chemistry Ø Takes care of “self-healing” How good is transport? Not as big an issue because lifetimes are long!

Other issues Changes in anthropogenic N 2 O emissions will affect: (a)the estimated date for the recovery of the ozone layer; (b)imply that the use of a single parameter such as Equivalent Effective Stratospheric Chlorine, EESC, to estimate the recovery of the ozone layer should be reevaluated; (c)implications for the recovery of the polar ozone hole that might differ from that of global ozone; (d)Calls in to question the “baseline” for ozone recovery. N 2 O could be an unintended byproduct of enhanced crop growth for biofuel production or iron fertilization to mitigate CO 2 emissions. Such an enhancement would lead to the unintended ‘indirect’ consequence of ozone layer depletion and increased climate forcing from N 2 O produced for alternative fuel used to curb global warming For history: Ozone depletion by anthropogenic N 2 O is roughly the same as that 24 from the original projections for 500 US supersonic transport SSTs.

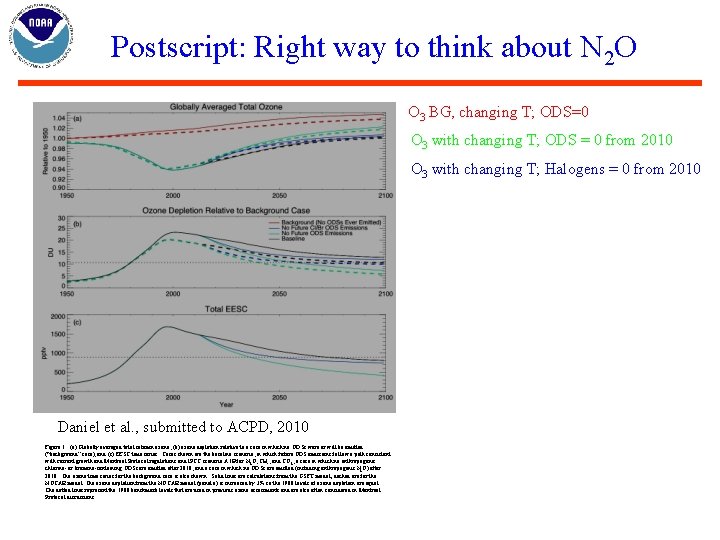
Postscript: Right way to think about N 2 O O 3 BG, changing T; ODS=0 O 3 with changing T; ODS = 0 from 2010 O 3 with changing T; Halogens = 0 from 2010 Daniel et al. , submitted to ACPD, 2010 Figure 1. (a) Globally averaged total column ozone, (b) ozone depletion relative to a case in which no ODSs were or will be emitted (“background” case), and (c) EESC time series. Cases shown are the baseline scenario, in which future ODS emissions follow a path consistent with current growth and Montreal Protocol regulations and IPCC scenario A 1 B for N 2 O, CH 4, and CO 2, a case in which no anthropogenic chlorine- or bromine-containing ODSs are emitted after 2010, and a case in which no ODSs are emitted (including anthropogenic N 2 O) after 2010. The ozone time series for the background case is also shown. Solid lines are calculations from the GSFC model; dashed are for the NOCAR model. The ozone depletion from the NOCAR model (panel a) is increased by 3% so the 1980 levels of ozone depletion are equal. The dotted lines represent the 1980 benchmark levels that are used in previous ozone assessments and are also often considered in Montreal Protocol discussions.

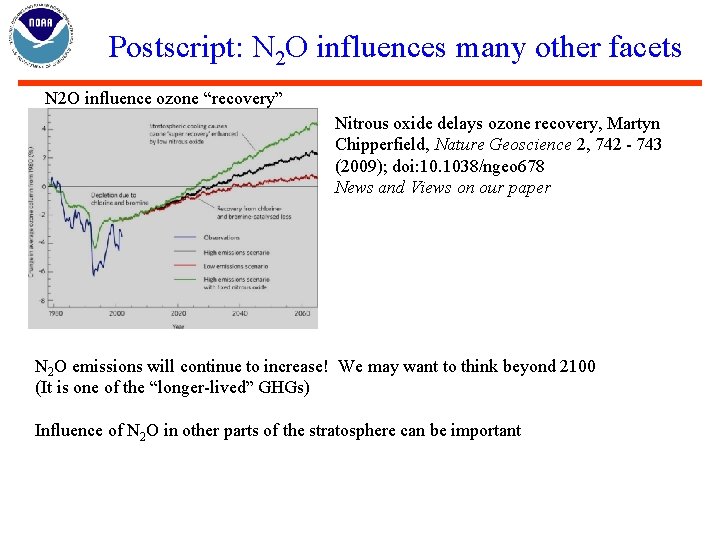
Postscript: N 2 O influences many other facets N 2 O influence ozone “recovery” Nitrous oxide delays ozone recovery, Martyn Chipperfield, Nature Geoscience 2, 742 - 743 (2009); doi: 10. 1038/ngeo 678 News and Views on our paper N 2 O emissions will continue to increase! We may want to think beyond 2100 (It is one of the “longer-lived” GHGs) Influence of N 2 O in other parts of the stratosphere can be important