Gas Cylinders Description Anaesthetists use gas cylinders every











- Slides: 16

Gas Cylinders Description Anaesthetists use gas cylinders every day as part of their clinical practice. This session examines the physical components of the gas cylinders, analyses the pressure changes that exist in oxygen and nitrous oxide cylinders, and explores Entonox (BOC Healthcare) cylinders.

Session introduction Learning objectives: by the end of this session you will be able to: Identify the physical composition of cylinders, cylinder valves and their storage. Interpret the key aspects of Pin Index System. Recognize and manage pressure changes in Oxygen and Nitrous Oxide cylinders. Define the features of Entonox cylinders.

introduction • • • Medical gases and vapours used in hospitals are often stored in cylinders under pressure. The cylinders which hold such gases are made of thin walled steel which is designed and fabricated to withstand considerable internal pressure. However to reduce weight, a recently introduced design means that aluminum alloy rather than steel is now commonly used for cylinder construction. On the steel cylinder shown, note components such as the valves, plastic disc and neck. The year the cylinder was last examined can be identified from the shape and colour of the disc

oxygen • • • Oxygen has been used in clinical practice for more than 200 years. It is probably the most widely prescribed medication in prehospital and hospital environments. If appropriately used is life saving and part of the first line treatment in many critical conditions. It is produced by the separation of air using cryogenic distillation and stored in cylinders at a pressure of 13700 KPa. because oxygen is a gas, there is a linear and proportional reduction in oxygen cylinder pressure as the cylinder empties at a constant temperature.

Nitrous oxide • Nitrous oxide is used as an adjunct to other anaesthetic drugs and also as an analgesic. It is stored in a liquid phase with its vapor at a pressure of 4400 KPa. • The nitrous oxide cylinder is only partially filled because in liquid form, it is less compressible than as a gas, the amount of filling is known as the filling ratio. • As the cylinder contains liquid and vapor. Initially the pressure remain constant as more vapor is produced to replaced what has been used. Once all the liquid has been evaporated, the pressure in the cylinder decreases. • The cylinder pressure does not accurately indicate the cylinder contents. The temperature in such a cylinder can decreases due to the loss of the latent heat of vaporization which lead to the formation of ice on the out side of the cylinder.

Nitrous oxide • Nitrous oxide cylinders are only partially filled with liquid in order to reduce the risk of a dangerous increase in pressure, an increase in the ambient temperature can lead to an explosion. • In UK, the filling ratio for nitrous oxide and carbon dioxide is 0. 75. • In hotter climates, the filling ratio is reduced to 0. 67.

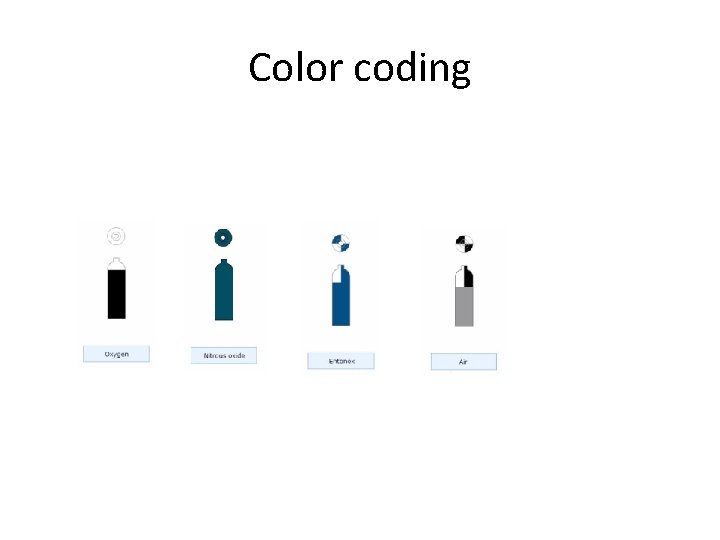
Cylinder size and color coding • • For patient safety and from a practical point of view, different medical gas cylinders types have various distinguishing features, such as their size and color. Cylinders are manufactured in different sizes depending on the gas they contain. These cylinder sizes are labelled from A to J. however, sizes A and H are not used for medical gases. Those attached to the anaesthetic machine are usually size E, while size J cylinders are commonly used for cylinder manifold.

Color coding

Cylinder markings and engravings • • • Several important distinguishing marks are engraved by the manufacturers on the exterior of all medical cylinders. These include: Test pressure: is employed to test the integrity of the cylinder and its ability to withstand extremely high pressures – the cylinder is subjected to pressures of approximately 22000 KPa. Dates of tests performed: every five years, cylinders in use are checked and tested by manufacturer. Chemical formula of cylinder contents: in addition to colour coding the cylindersthe chemical formula confirms the contents of the cylinder. Tare weight: this is the weight of nitrous oxide cylinder when empty.

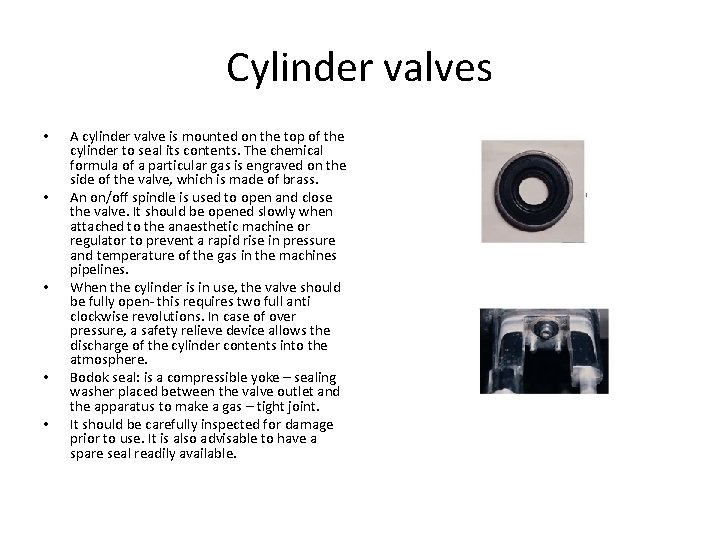
Cylinder valves • • • A cylinder valve is mounted on the top of the cylinder to seal its contents. The chemical formula of a particular gas is engraved on the side of the valve, which is made of brass. An on/off spindle is used to open and close the valve. It should be opened slowly when attached to the anaesthetic machine or regulator to prevent a rapid rise in pressure and temperature of the gas in the machines pipelines. When the cylinder is in use, the valve should be fully open- this requires two full anti clockwise revolutions. In case of over pressure, a safety relieve device allows the discharge of the cylinder contents into the atmosphere. Bodok seal: is a compressible yoke – sealing washer placed between the valve outlet and the apparatus to make a gas – tight joint. It should be carefully inspected for damage prior to use. It is also advisable to have a spare seal readily available.

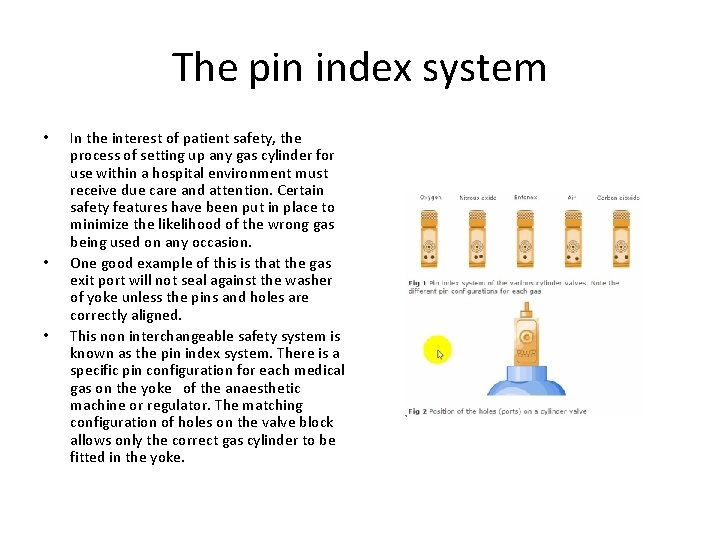
The pin index system • • • In the interest of patient safety, the process of setting up any gas cylinder for use within a hospital environment must receive due care and attention. Certain safety features have been put in place to minimize the likelihood of the wrong gas being used on any occasion. One good example of this is that the gas exit port will not seal against the washer of yoke unless the pins and holes are correctly aligned. This non interchangeable safety system is known as the pin index system. There is a specific pin configuration for each medical gas on the yoke of the anaesthetic machine or regulator. The matching configuration of holes on the valve block allows only the correct gas cylinder to be fitted in the yoke.

Entonox • • Entonox, a gas mixture of 50% oxygen and 50% nitrous oxide by volume, can be used to provide analgesia. It is compressed into cylinders to a pressure of 13700 KPa. A two stage pressure demand regulator is attached to the cylinder. As the patient inhales through the mask or mouthpiece, gas flow is allowed to occur. Gas flow ceases at the end of an inspiratory effort.

Entonox The Poynting Effect: The liquefaction and separation of the two component gases of entonox can occur if a cylinders temperature is decreased to below -5. 5 C ( the poynting effect). This result in: At the bottom of the cylinder, a liquid mixture containing mostly nitrous oxide with about 20% oxygen dissolved in it. Above the liquid, a gas mixture of high oxygen concentration. When used at a constant flow rate, a gas with a high concentration of oxygen is supplied first. This is followed by a gas of decreasing oxygen concentration as the liquid evaporates. This may lead to the supply of hypoxic mixtures with less than 20% oxygen as the cylinder empties. This separation and liquefaction can be reversed by re – warming the cylinder and mixing its contents by inverting it repeatedly.

Entonox Avoiding entonox separation: Cylinders are stored horizontally for about 24 hours at temperature of about 5 C or above before use, to increase the area available for diffusion. If the contents are well mixed by repeated inversion, cylinders can be used sooner than 24 hours. Large cylinders have a dip tube with it ending in the liquid phase. this result in the liquid being used first, preventing the delivery of an oxygen concentration of less than 20 %.

Cylinder storage and maintenance • • Because of the inherit dangers associated with working in the vicinity of pressurized cylinders, further safety procedures must be rigorously adhered to for the maintenance and storage of gas cylinders. Full cylinders should be separated from empty ones to avoid accidents. This is to avoid the risk of disrupting patient gas supply by accidentally connecting an empty cylinder. Size F, G and J cylinders should be stored upright to avoid damage to the valves. Size C, D and E cylinders can be stored horizontally on shelves made of a material that does not damage the surface of the cylinders, usually tember.

Session key points • • Gas cylinders are made of thin walled molybdenum steel to withstand high pressures. They are made in different sizes. Oxygen cylinders contain gas whereas nitrous oxide cylinder contain a mixture of liquid and vapour. In the UK, they are 75% filled with liquid nitrous oxide ( filling ratio). At a constant temperature, the pressure in a gas cylinder decreases linearly and proportionally as the cylinder empties. This is not true in a cylinder containing liquid/ vapour. Gas cylinder are colour coded and display labelling and marking. They undergo regular testing and checking. A cylinder valve is mounted on the neck of the cylinder which acts as on/off device for the discharge of the contents. The pin index system prevent cylinder identification errors. Entonox is a gas mixture of 50% oxygen and 50% nitrous oxide by volume.