Neoplasia 6 Dr Heym Awad FRCPath Cyclins and




























- Slides: 41

Neoplasia 6 Dr Heym Awad FRCPath

Cyclins and cyclin dependent kinases

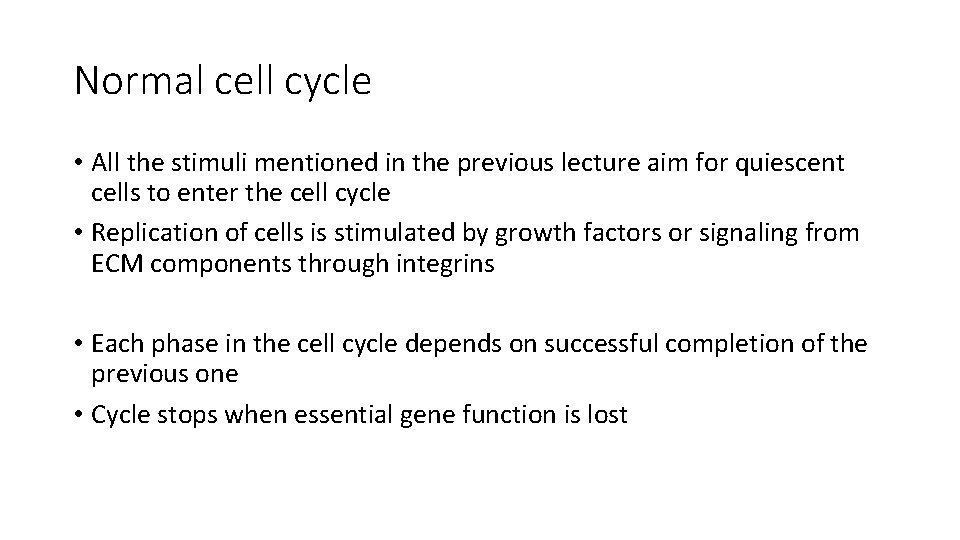
Normal cell cycle • All the stimuli mentioned in the previous lecture aim for quiescent cells to enter the cell cycle • Replication of cells is stimulated by growth factors or signaling from ECM components through integrins • Each phase in the cell cycle depends on successful completion of the previous one • Cycle stops when essential gene function is lost

• G 1 to S transition is critical because if this checkpoint is passed the cells are committed for DNA replication • G 1 to S is called the restriction point

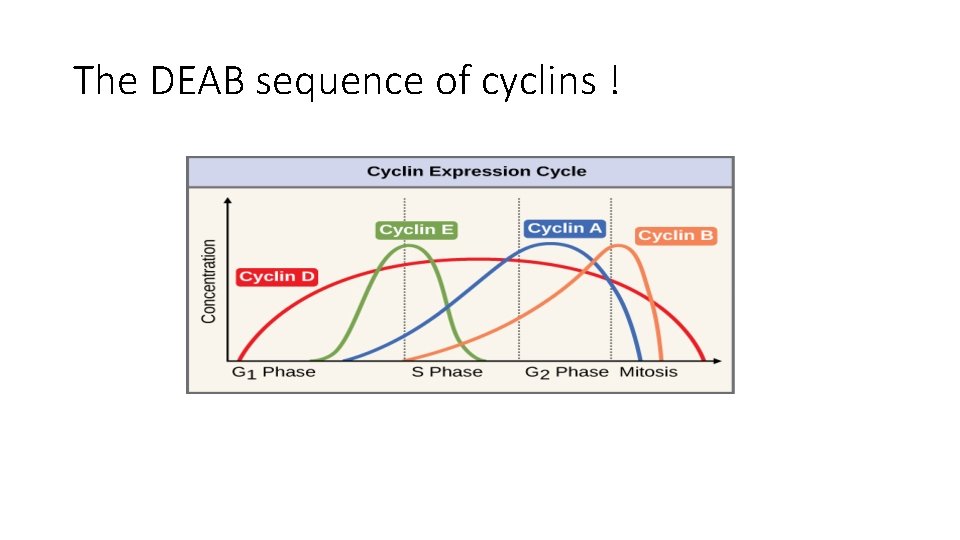
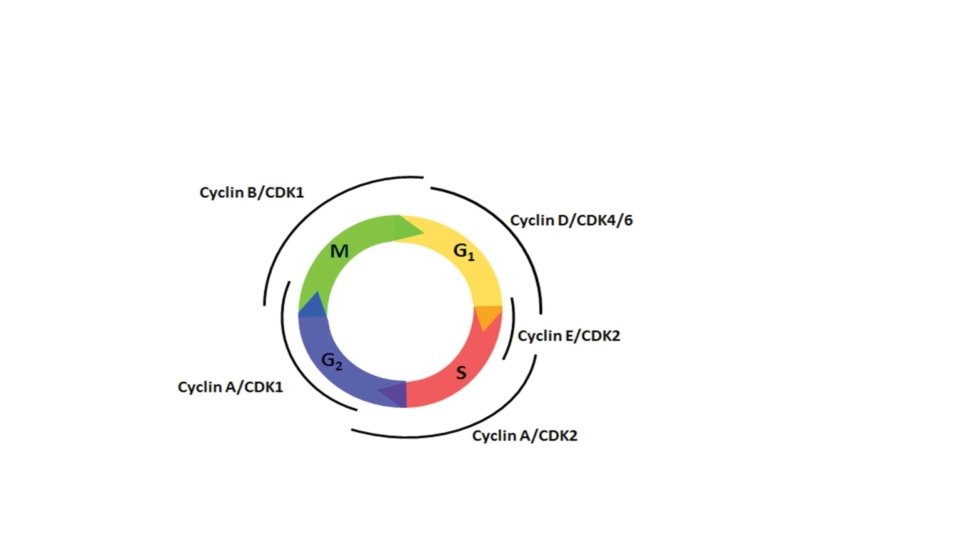
• Progression through cell cycle, especially G 1 -S is regulated by proteins called cyclins • Cyclins activate kinases CDK (cyclin dependent kinases) • Cyclin and CDK form complexes that phosphorylate target proteins that drive the cell through the cell cycle. • Cyclins : D, E, A , B (they appear in this sequence

The DEAB sequence of cyclins !

• SO: cyclin/CDK complexes cause proliferation. • Theses are inhibited by cyclin dependent kinase inhibitors (CDKI) • CDKI important for enforcing the checkpoints and delaying cell cycle.


• Checkpoints important to discover DNA damage and prevent cells with DNA damage from continuing cell cycle • G 1 -S checkpoint monitors integrity of chromosomes before replicating • G 2 -M checks DNA integrity after replication to decide if cells can safely go into mitosis

• When there is DNA damage the checkpoints are activated. . They delay the cell cycle and trigger DNA repair • If damage is severe: apoptosis or senescence are stimulated.

CDKI • Some inhibit CDK broadly p 21, p 27, p 57 • Others are specific p 15, p 16, p 18, p 19 • (you don’t need to remember which is which!!)

• Mutations causing increased cyclins or CDK cause self sufficiency in growth signals. • Mutations inhibiting CDKI will cause increased growth. • Examples: cyclin D is activated in several tumors mainly lymphomas.

Second hallmark of cancer • 2. insensitivity to growth inhibitors • Growth inhibition is achieved by tumor suppressor genes • Loss or decreased functions of tumor suppressor genes is essential to cause cancer

• RB gene= retinoblastoma gene = governor of cell cycle

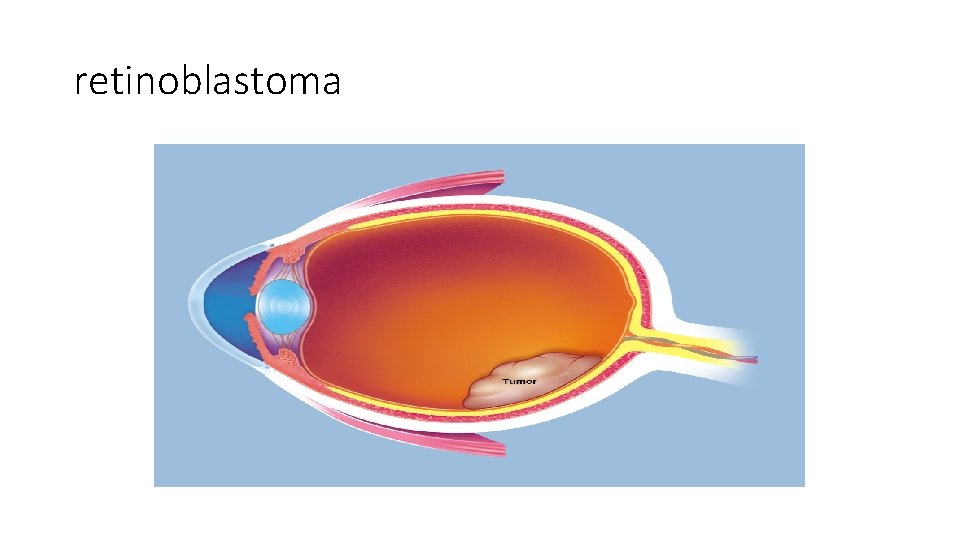
retinoblastoma • Rare childhood tumor • 60 % of cases are sporadic, 40% familial • Predisposition to develop the tumor is inherited as autosomal dominant trait • To develop retinoblastoma: two hit hypothesis

retinoblastoma

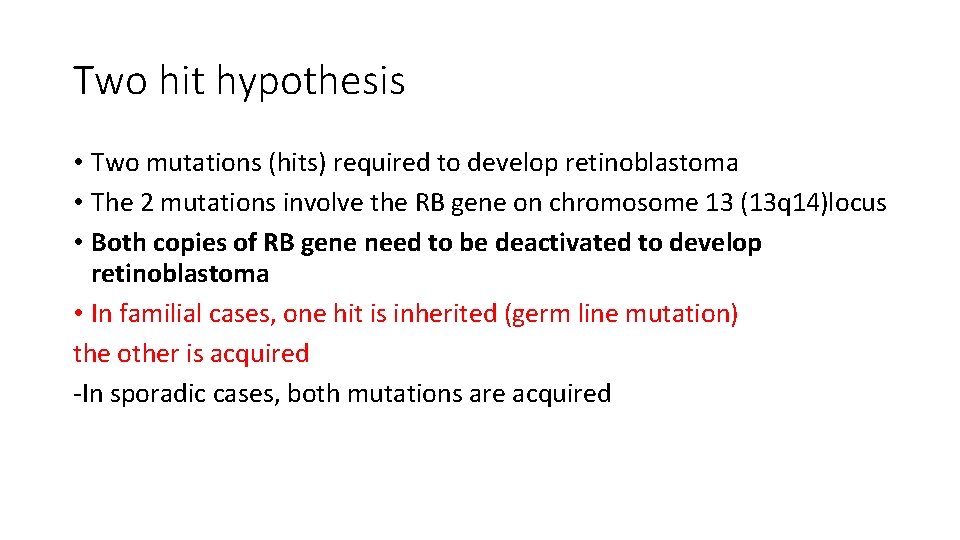
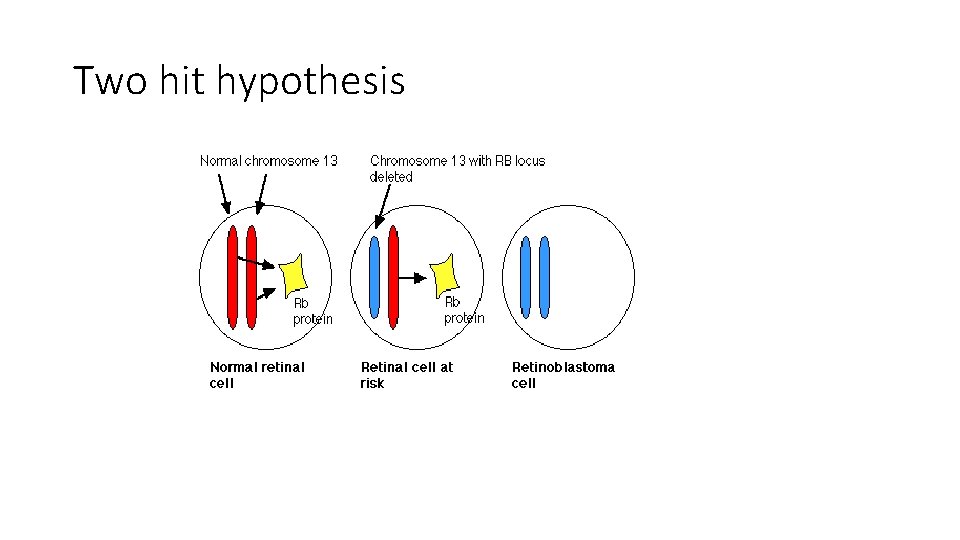
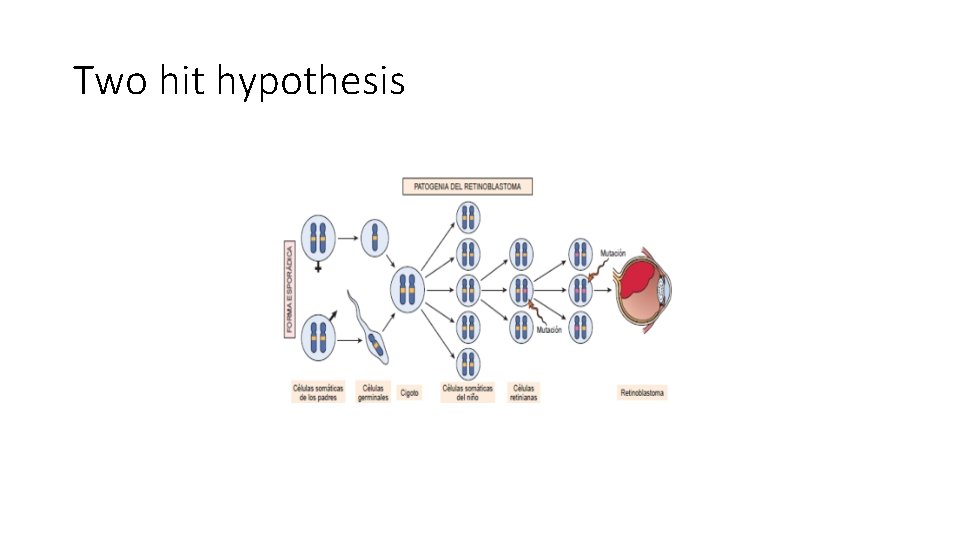
Two hit hypothesis • Two mutations (hits) required to develop retinoblastoma • The 2 mutations involve the RB gene on chromosome 13 (13 q 14)locus • Both copies of RB gene need to be deactivated to develop retinoblastoma • In familial cases, one hit is inherited (germ line mutation) the other is acquired -In sporadic cases, both mutations are acquired

• In familial cases , one single somatic mutation is needed. . So dominant pattern of inheritance. • NOTE: retinoblastoma disease follows autosomal dominant inheritance. Because the susceptibility of the disease is what is inherited. BUT, RB gene is a recessive gene.

Two hit hypothesis

Two hit hypothesis

• People with inherited RB have increased risk of other cancers. . Mainly osteosarcomas and soft tissue sarcomas • Homozygous loss (both copies lost) of RB gene can be seen in many cancers like breast, bladder…

• A cell heterozygous in RB locus is not neoplastic ( one normal and one abnormal allele) • The two hits are essential for neoplastic transformation

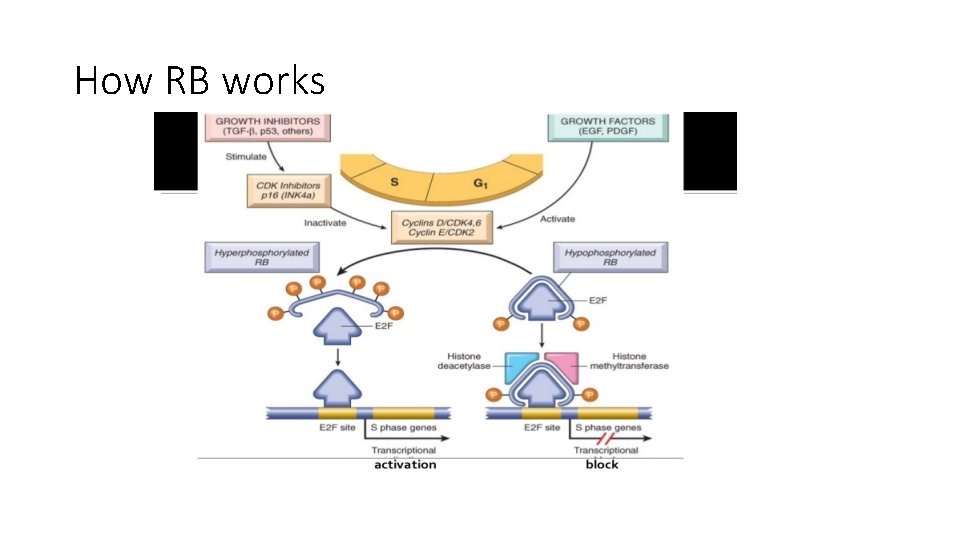
RB gene • RB gene product is a DNA binding protein expressed in all cells • This protein has two forms: active hypophsphorylated state and inactive hyperphosphorylated • Bb regulates G 1/S checkpoint

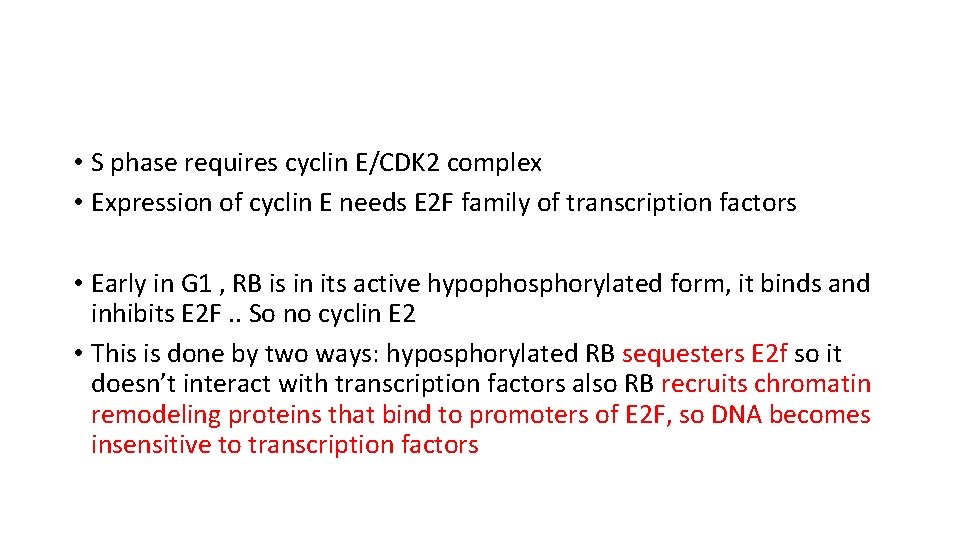
• S phase requires cyclin E/CDK 2 complex • Expression of cyclin E needs E 2 F family of transcription factors • Early in G 1 , RB is in its active hypophosphorylated form, it binds and inhibits E 2 F. . So no cyclin E 2 • This is done by two ways: hyposphorylated RB sequesters E 2 f so it doesn’t interact with transcription factors also RB recruits chromatin remodeling proteins that bind to promoters of E 2 F, so DNA becomes insensitive to transcription factors

• Mitogenic signals. . lead to cyclin d expression and activate cyclin D/CDK 4/6 complexes. . These complexes phosphorylate RB. . • Phosphorylated RB is inactivate … this leads to release of E 2 F • E 2 F now free and can cause transcription of cyclin E. . • Cyclin E stimulates DNA replication • So cells enter S phase • Once in s phase cells are committed to division. . They don’t need additional growth signals • In M phase phosphate removed from RB , so it goes back to its inactive state.

How RB works

• E 2 F is not the only transcription factor targeted by RB • Rb stimulates myocyte, adipocyte and other cell specific transcription fatcors… so important for G 0 -G 1 with differentiation

• Rb is important for tumerogenesis but it is not mutated in all cancers • If it is not mutated, other gene mutations mimicking RB mutation must play a role • Mutations in genes affecting RB phosphorylation: Mutatiomnal activation of cdk 4 or overexpression of cyclin D favour cell proliferation by inactivating RB

NOTE • Loss of nomal cell cycle control is found in all tumors through mutations of RB, cyclin D, CDK 4 or CDKN 2 A (which is a CDKI)

• Some virus like HPV have protein E 7 which binds to the hypophsphorylated RB so prevents it from inhibiting E 2 F • So RB is functionally deleted

TP 53 gene: the guardian of the genome • Tp 53 is one of the most commonly mutated genes in cancer • It encodes p 53 protein P 53 causes growth inhibition by three mechanisms 1. Temporary cell cycle arrest: quiescence 2. permenant cell cycle arrest: senescence 3. triggers apoptosis

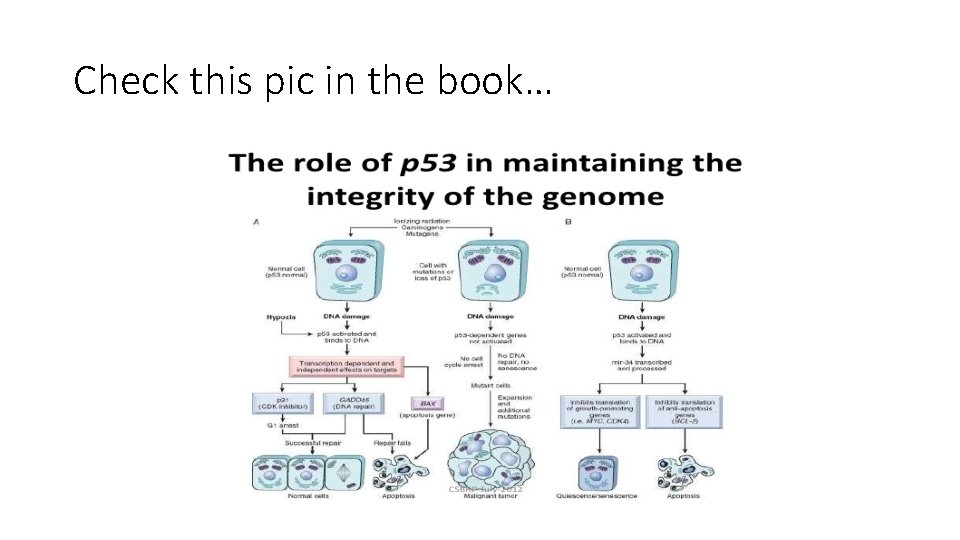
Check this pic in the book…

• P 53 monitors internal stress whereas RB senses external signals • P 53 is triggered by several stresses: anoxia, inappropriate oncogene activity (MYC or RAS) or DNA damage.

• In non-stressed healthy cell, p 53 is short lived: 20 minutes because it binds MDM 2 which is a protein that targets it for destruction • When cells are stressed. . sensors that include protein kinases are activated (ATM is one of these kinases) • These activated kinases catalyze post translational modifications of p 53 and release it from MDM 2 • Now p 53 has longer life span and can drive transcription of certain genes. . hundreds of them

Genes transcribed by p 53 • Suppress growth by three mechanisms • 1. mediating cell cycle arrest. This occurs late in G 1. caused by p 53 dependent transcription of CDK 1 gene= p 21= CDKNIA • P 21 protein inhibit cyclin/CDK complexes and prevent phosphorylation of RB • So cell is arrested in G 1 • Pause to repair any DNA damage

• P 53 also induces expression of DNA damage repair genes • If DNA is repaired successfully , p 53 upregulates transciption of MDM 2. . Destruction of p 53. . Removal of the block on cell cycle. • If DNA not repaired p 53 makes cells enter apoptosis or senescence

Senescence by p 53 • Senescence needs activation f p 53 and or RB and expression of their mediators like CDKI • Mechanisms of senescence unclear but seem to involve global chromatin change, which permanent change gene expression

P 53 induced apoptosis • Induced by pro-apoptotic genes including BAX and PUMA

• P 53 also represses proliferative and anti-apoptotic genes (bcl 2) • ? P 53 is a transcriptional activator so how could it repress certain gene expression • Answer by mi. RNAs

• More than 70% of human cancers have mutated TP 53 • Both copies of the gene need to be lost for cancer to develop • Mostly somatic • Rare li Fraumini syndrome: inherit defect in one allele. . More predisposition to cancer (Sarcoma, breast carcinoma , leukemia and brain tumor)

• P 53 can become nonfunctional by some DNA viruses • HPV, Hep B, EBV. . Proteins can bind to p 53 and deactivate it • Note p 53 activated by phosphorylation
 Cell cycle graph
Cell cycle graph Awad mataria
Awad mataria Khaled awad md
Khaled awad md Define exemplum
Define exemplum Gott der stadt
Gott der stadt Frcpath part 1 microbiology
Frcpath part 1 microbiology Frcpath examination
Frcpath examination Frcpath eligibility
Frcpath eligibility Clinical features of neoplasia
Clinical features of neoplasia Burkitt lymphoma
Burkitt lymphoma Patologia
Patologia Malignancy
Malignancy Malignant neoplasm
Malignant neoplasm Cin
Cin Ciclo emorragico
Ciclo emorragico Mamria
Mamria Kenneth loaiciga
Kenneth loaiciga Polyp
Polyp Multiple endocrine neoplasia type 2
Multiple endocrine neoplasia type 2 Que es una neoplasia
Que es una neoplasia Neoplasia literally means
Neoplasia literally means Neoplasia testicular
Neoplasia testicular Anovulatory bleeding
Anovulatory bleeding New growth meaning
New growth meaning Purple green and blue yellows
Purple green and blue yellows Willaim blake
Willaim blake Romeo and juliet vs west side story
Romeo and juliet vs west side story Taller stronger little sister
Taller stronger little sister How to write five centavos
How to write five centavos Red+orange+yellow+green+blue+indigo+violet=
Red+orange+yellow+green+blue+indigo+violet= Your teacher is giving you a test worth 100 points
Your teacher is giving you a test worth 100 points Young and dyslexic poem
Young and dyslexic poem Compare and contrast maynard jackson and andrew young
Compare and contrast maynard jackson and andrew young West yorkshire and harrogate health and care partnership
West yorkshire and harrogate health and care partnership Compare and contrast transverse and longitudinal waves
Compare and contrast transverse and longitudinal waves High tide
High tide Dependent variable
Dependent variable Lesson 5 verbs action (transitive/intransitive) answer key
Lesson 5 verbs action (transitive/intransitive) answer key What are life roles?
What are life roles? Records and reports in nursing
Records and reports in nursing Prohibited route in transportation problem means
Prohibited route in transportation problem means Compare and contrast cold wave and wind chill factor
Compare and contrast cold wave and wind chill factor