NAVIGATING THE FDA Vivian K Dullien Ph D



































- Slides: 38

NAVIGATING THE FDA Vivian K. Dullien, Ph. D FDA, Clinical and Business Consultant Dullien Associates, LLC Boulder, Colorado

Meet with FDA early in process and keep in touch. NAVIGATING THE FDA Vivian K. Dullien, Ph. D 2

Before you meet, do your homework. NAVIGATING THE FDA Vivian K. Dullien, Ph. D 3

What are you? • Diagnostic • Device • Drug • Biologic • Combination NAVIGATING THE FDA Vivian K. Dullien, Ph. D 4

What disease? NAVIGATING THE FDA Vivian K. Dullien, Ph. D 5

What for? • Screening? • Diagnosing? • Monitoring? • Treating? NAVIGATING THE FDA Vivian K. Dullien, Ph. D 6

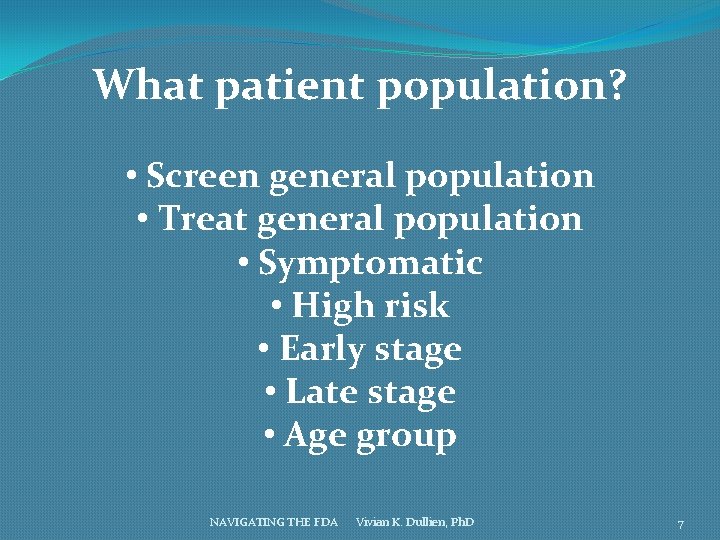
What patient population? • Screen general population • Treat general population • Symptomatic • High risk • Early stage • Late stage • Age group NAVIGATING THE FDA Vivian K. Dullien, Ph. D 7

If device or diagnostic, is there a predicate? Comparison to predicate – where similar, where not NAVIGATING THE FDA Vivian K. Dullien, Ph. D 8

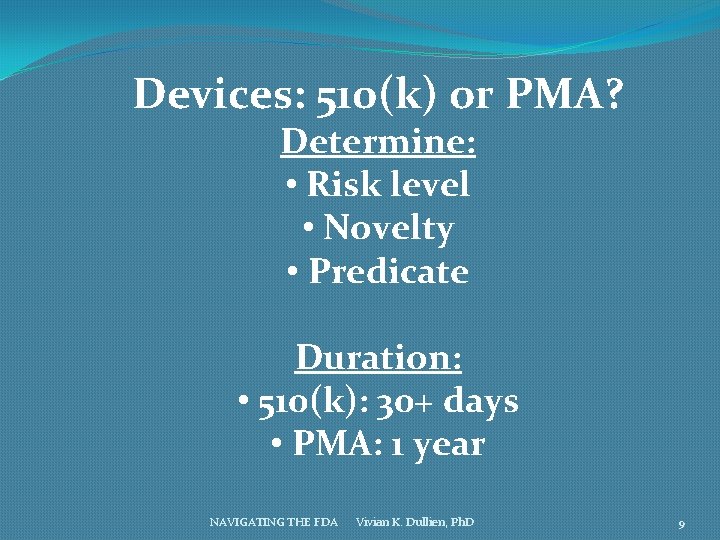
Devices: 510(k) or PMA? Determine: • Risk level • Novelty • Predicate Duration: • 510(k): 30+ days • PMA: 1 year NAVIGATING THE FDA Vivian K. Dullien, Ph. D 9

Drug or device? • The driver in the FDA process – “Indications for Use” • Part of product label NAVIGATING THE FDA Vivian K. Dullien, Ph. D 10

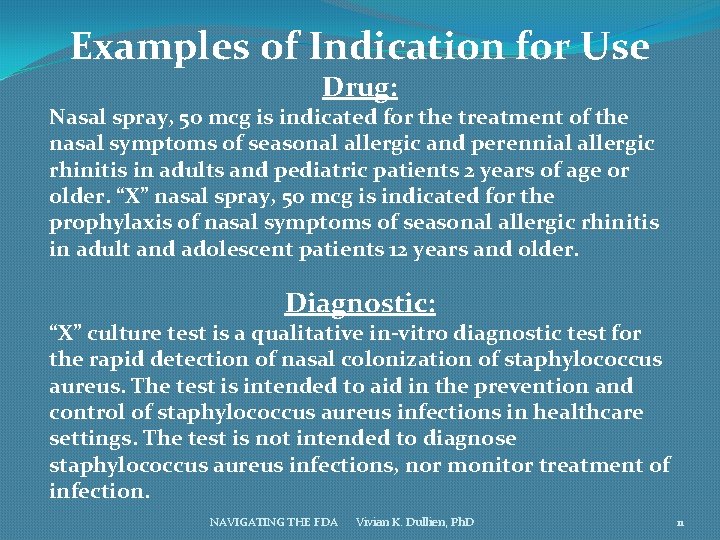
Examples of Indication for Use Drug: Nasal spray, 50 mcg is indicated for the treatment of the nasal symptoms of seasonal allergic and perennial allergic rhinitis in adults and pediatric patients 2 years of age or older. “X” nasal spray, 50 mcg is indicated for the prophylaxis of nasal symptoms of seasonal allergic rhinitis in adult and adolescent patients 12 years and older. Diagnostic: “X” culture test is a qualitative in-vitro diagnostic test for the rapid detection of nasal colonization of staphylococcus aureus. The test is intended to aid in the prevention and control of staphylococcus aureus infections in healthcare settings. The test is not intended to diagnose staphylococcus aureus infections, nor monitor treatment of infection. NAVIGATING THE FDA Vivian K. Dullien, Ph. D 11

Product label can include: • Description (clinical pharmacology) • Clinical studies summary • Indication and usage • Warnings • Precautions • Adverse reactions • Contra indications • Dosage and administration • Information for patients NAVIGATING THE FDA Vivian K. Dullien, Ph. D 12

Your FDA studies are to support the claims in your “Indications for Use” NAVIGATING THE FDA Vivian K. Dullien, Ph. D 13

“Indications for Use” Will determine what you can say in marketing material once you have been approved NAVIGATING THE FDA Vivian K. Dullien, Ph. D 14

Study endpoints • How will you measure your safety and efficacy? • If device – agreement to predicate – what is your predicate? • What is the gold standard? • Are you an AID in diagnosing/treating or stand-alone? NAVIGATING THE FDA Vivian K. Dullien, Ph. D 15

Study endpoints: statistician’s inputs • Study power • N size • Methods to measure statistical significance NAVIGATING THE FDA Vivian K. Dullien, Ph. D 16

Survey the market • Review similar products on the market • Look at labels, Indications for Use, warnings and limitations NAVIGATING THE FDA Vivian K. Dullien, Ph. D 17

Ready to approach FDA • Phone, e-mail • Set up a face-to-face meeting: pre-IDE or pre-IND • Send in information before meeting • Include: product description, predicate comparison if applicable, suggestion on how to be classified, indications for use, protocol outline with study endpoints, suggest N size. If drug results of animal study safety, pilot/lab, feasibility results, statistical methods to be used • List of questions for FDA • Who will attend NAVIGATING THE FDA Vivian K. Dullien, Ph. D 18

FDA - organizational • On Internet (http: //www. fda. gov/opacom/7 org. html) • Office of the Commissioner: 301 -796 -5000 • Biologics: 301 -827 -0372 • Devices and Radiological Health: 240 -276 -3939 • Drug Evaluation: 301 -796 -5400 • Veterinary Medicine: 240 - 276 -9000 • Regulatory Affairs: 301 -827 -3101 • Food Safety and Applied Nutrition: 301 -436 -1600 NAVIGATING THE FDA Vivian K. Dullien, Ph. D 19

FDA meeting & post-meeting process • Get FDA feedback and take minutes • Send minutes to FDA for review • Incorporate FDA suggestions into your planned studies • Send protocol to FDA for review and comments before you start NAVIGATING THE FDA Vivian K. Dullien, Ph. D 20

Clinical devices: Investigation device exemption (IDE), unless exempt Need: • Protocol approved by IRB. If significant risk, the IDE must be approved by FDA • Informed consent from patient • Labeling for investigational use only • Required records and reports • Conduct under GCP (21 CFR 812, 50, 56, 54, 820) NAVIGATING THE FDA Vivian K. Dullien, Ph. D 21

IDE includes: • Device name and description • Device intended use • Sponsor contact information • Manufacturer information • Laboratory studies • Reports of animal studies • Prior publications • Investigational plan, including protocol, risk analysis, monitoring procedures, list of investigators, IRB’s, informed consents • Labeling • Description of methods, facilities and controls used for manufacture, processing, packing, storage, installation of device NAVIGATING THE FDA Vivian K. Dullien, Ph. D 22

IDE exempt: • Non-invasive • Does not require invasive sampling that presents significant risk • Does not introduce energy into a subject • Is not used as a diagnostic procedure, without confirmation of another medically established product or procedure NAVIGATING THE FDA Vivian K. Dullien, Ph. D 23

Phases of device studies • Feasibility • Pivotal • 510(k) • PMA NAVIGATING THE FDA Vivian K. Dullien, Ph. D 24

Pre-IDE process • Informal guidance meeting (before IDE submitted): help with development of protocol, supporting pre-clinical data. Can be telephone conference calls, video conference, or face-to-face • Formal guidance meeting: determination meeting – written request to review intended use, protocol, and type of valid scientific evidence required. A pre-IDE submission • Agreement meeting – written request to reach agreement with FDA regarding FDA’s review of the clinical protocol and investigational plan NAVIGATING THE FDA Vivian K. Dullien, Ph. D 25

Agreement meeting written request: • Detailed description of device • Detailed description of proposed conditions of use of device • Proposed plan (including clinical protocol) • Information regarding expected performance Agreement put in writing Keep in contact with FDA throughout process from informal to formal agreement meetings NAVIGATING THE FDA Vivian K. Dullien, Ph. D 26

Additional guidances can be found: • Early collaboration meetings • Goals and initiatives for the IDE program • Pre-IDE program: “Issues and Answers” • Search Internet and find guidances. • FDA has put guidances on the Internet for devices and drugs NAVIGATING THE FDA Vivian K. Dullien, Ph. D 27

Drug review steps 1. Pre-IND, animal testing 2. IND – outlines human testing. Meet with FDA before submitting IND reviewed by FDA and IRB 3. Phase 1: 20 -80 subjects 4. Phase 2: 12 -300 subjects 5. Phase 3: several hundred to 3000. Meet with FDA before Phase 3 6. Pre-NDA, meet with FDA before submitting NDA 7. Submit NDA 8. Review 9. Facilities inspected 10. Approvable or not approvable NAVIGATING THE FDA Vivian K. Dullien, Ph. D 28

• • • Pre-clinical testing in animals Assessing toxicity in single doses of increasing strength, 2+ species Different routes of administration Determine ED 50, LD 50, duration of effects Subjects autopsied for cause of death Sub-acute toxicity 3 or more routes of administration 3 different dose levels, 2+ species, observed 2 – 12 weeks Estimate what human dosages will be Especially note liver, kidneys and nervous system Chronic toxicity 3 – 24 months At least 6 months before human studies Carcinogenic effects, 6 months, 2+ species, same route of administration as humans Teratogenic effects NAVIGATING THE FDA Vivian K. Dullien, Ph. D 29

IND contents include: • Pre-clinical toxicology and pharmacology results • Phase 1 protocols • Drug facilities, processes, articles used in manufacturing, processing, packaging, and storing drugs for humans • Chemistry, manufacturing and control information • Previous experience with human subjects NAVIGATING THE FDA Vivian K. Dullien, Ph. D 30

• Phase 1 Drug clinical stages - healthy volunteers - Side effects, how drug is metabolized and excreted - Safety • Phase 2 - Patients - Safety - Effectiveness – preliminary information if drug works in humans with the disease • Phase 3 - large safety and efficacy studies - different dosages, drug combinations and populations NAVIGATING THE FDA Vivian K. Dullien, Ph. D 31

Timelines – clinical testing of drugs Range: 2 – 10 years (average 5 – 7 years) • Phase 1 1 + years • Phase 2 2 + years • Phase 3 3 + years NDA usually 100, 000 pages in length, takes at least 6 months to review. Average 2 years (range 2 months to 7 years. ) NAVIGATING THE FDA Vivian K. Dullien, Ph. D 32

Quality of clinical data • IRB approval, informed consent • Study conducted according to protocol • adverse events recorded • subsets met the inclusion/exclusion criteria NAVIGATING THE FDA Vivian K. Dullien, Ph. D 33

Reviewing applications for drug or device FDA review team - medical doctors, chemists, statisticians, microbiologists, pharmacologists and other experts - determine if drug/device is safe and effective for its proposed use Each reviewer prepares written evaluations that are considered by team leaders, division directors, office directors, depending on type of application • Sometimes FDA calls upon advisory committees NAVIGATING THE FDA Vivian K. Dullien, Ph. D 34

Issues that can arise • Clinical data not supportive of indications for use – need more data • Inadequacy on how people respond to various dosages – need more data • “Approvable” – probably be approved, provided certain issues get resolved • Labeling • Safety issues • Manufacturing issues – can delay or deny application. Product that is going to be marketed is the same product that was tested • Depending on FDA action – meet to discuss deficiencies, ask for hearing, correct deficiencies, submit new information or withdraw application NAVIGATING THE FDA Vivian K. Dullien, Ph. D 35

Device or drug Continuous interaction between FDA and company during review process NAVIGATING THE FDA Vivian K. Dullien, Ph. D 36

It all comes down to this: The package insert NAVIGATING THE FDA Vivian K. Dullien, Ph. D 37

Thank you! Vivian K. Dullien, Ph. D. Dullien Associates, LLC Dullien. V@comcast. net (303) 499 -6777 NAVIGATING THE FDA Vivian K. Dullien, Ph. D 38
 Digital landscape model
Digital landscape model Navigating the art world
Navigating the art world Primatic compass
Primatic compass Aws iaa
Aws iaa Math bearings
Math bearings Regions of the body
Regions of the body Navigating gdpr compliance on aws
Navigating gdpr compliance on aws Vivian luna
Vivian luna Vivian burey marshall
Vivian burey marshall Joe burrus
Joe burrus Class b mundic
Class b mundic Vivian chum chum
Vivian chum chum Vivian is using a search engine to find photos
Vivian is using a search engine to find photos Characters in a lesson before dying
Characters in a lesson before dying Los mapuches como vivian
Los mapuches como vivian Sonhar com onça pintada
Sonhar com onça pintada Vivian siegel
Vivian siegel Dr vivian ho
Dr vivian ho Vivian verbo
Vivian verbo Dr vivian lin
Dr vivian lin Johnny cash first wife ethnicity
Johnny cash first wife ethnicity Illuminated manuscript font
Illuminated manuscript font Viviandthemagicisland
Viviandthemagicisland Vivian medeiros
Vivian medeiros Vivian mai
Vivian mai Vivian ekchian
Vivian ekchian Como se vestian los comechingones
Como se vestian los comechingones Homo habilis periodo y características
Homo habilis periodo y características Vivian hutchinson
Vivian hutchinson Một số thể thơ truyền thống
Một số thể thơ truyền thống Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Sơ đồ cơ thể người
Sơ đồ cơ thể người Lp html
Lp html Các số nguyên tố
Các số nguyên tố Tư thế ngồi viết
Tư thế ngồi viết đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân Thang điểm glasgow
Thang điểm glasgow