Naming Polyatomic Ions and Acids Oxyanions n Oxyanions
















- Slides: 16

Naming Polyatomic Ions and Acids

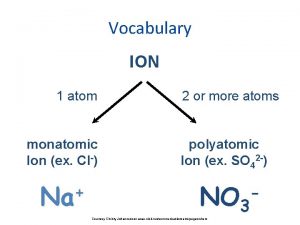
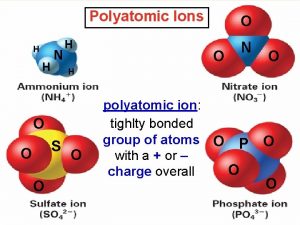
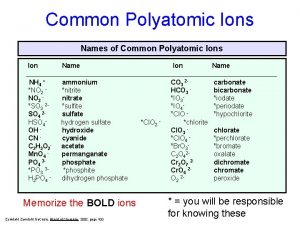
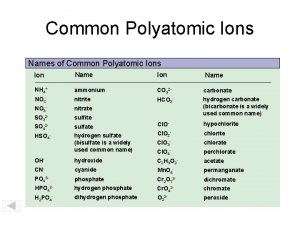
Oxyanions n Oxyanions- negative ions containing oxygen. n These have the suffix “-ate” or “-ite” n “-ate” means it has more oxygen atoms bonded, “-ite” has less n For example n SO 42 - sulfate n SO 32 - sulfite

Oxyanions n Oxyanions may contain the prefix “hypo- ”, less than, or “per-”, more than. n For example n Cl. O 4 Perchlorate n Cl. O 3 Chlorate n Cl. O 2 Chlorite n Cl. OHypochlorite

Acids n Certain compounds produce H+ ions in water, these are called acids. n You can recognize them because the neutral compound starts with “H”. n For example HCl, H 2 SO 4, and HNO 3. n All acids are like ionic compounds but the metal is replaced with H+ n It will be H +halogens or H + polyatomic ions

Naming acids n Does it contain oxygen? n If it does not, it gets the prefix “hydro-” and the suffix “-ic acid” n HCl n Hydrochloric acid n HF n Hydrofluoric acid n HCN n Hydrocyanic acid

Naming Acids n If it does contain an oxyanion, then replace the ending. n If the ending was “–ate”, add “-ic acid” n If the ending was “–ite”, add “-ous acid” n H 2 SO 4 Sulfuric Acid n H 2 SO 3 Sulfurous Acid

Examples n HNO 3 n Nitric acid n HCl. O n Hypochlorous acid n HCl. O 2 n Chlorous acid n HCl. O 4 n Perchloric acid n H 2 CO 3 n Carbonic acid n HF n Hydrofluoric acid

Nomenclature (naming) of Covalent compounds

Determining the type of bond n First, determine if you have an ionic compound or a covalent compound. n A metal and a nonmetal will form an ionic bond. n Compounds with Polyatomic ions form ionic bonds. n Nonmetals bonding together or Nonmetals and a metalloid form covalent bonds.

Covalent bonding is very similar to ionic naming n You always name the one that is least electronegative first (furthest from fluorine) n Most electronegative last, and gets the suffix “-ide”.

Covalent bonding is very different from ionic naming n Ionic names ignored the subscript because there was only one possible ratio of elements. n Covalent gives several possibilities so we have to indicate how many of each atom is present in the name

Prefixes you have to know prefix meaning *mono- 1 hex- 6 di- 2 hept- 7 tri- 3 oct- 8 tetr- 4 non- 9 pent- 5 dec- 10 * the first atom named does not get the prefix “mono-”, it just keeps its original name!

Examples n CO n carbon monoxide n CO 2 n carbon dioxide n NI 3 n nitrogen triiodide n P 4 O 6 n tetraphosphorus hexoxide

Continuing n I 4 O 9 n tetriodine nonoxide n S 2 F 10 n disulfur decafluoride n IF 7 n Iodine heptafluoride n Si 2 Cl 6 n disilicon hexachloride

Something to be wary of n Many chemicals have “common names” because they have been in use for so long n like H 2 O 2 n following naming rules it is… n dihydrogen dioxide n commonly it is hydrogen peroxide.

Homework n Go to http: //www. DHMO. org and write a 2 paragraph reaction to the site citing actual information presented in the site. Not just an angry rant, but an intelligent opinion. n Pay attention to what you just learned! n Due by next Wednesday
 Ionic compounds containing polyatomic ions
Ionic compounds containing polyatomic ions Whats a monatomic ion
Whats a monatomic ion Monatomic ion definition chemistry
Monatomic ion definition chemistry Monatomic formula
Monatomic formula 89 common polyatomic ions
89 common polyatomic ions Multivalent metals definition
Multivalent metals definition Polyatomic ion list
Polyatomic ion list 6 polyatomic ions
6 polyatomic ions Lewis structure for polyatomic ions
Lewis structure for polyatomic ions How to memorize polyatomic ions song
How to memorize polyatomic ions song Perphosphate polyatomic ion
Perphosphate polyatomic ion 6 polyatomic ions
6 polyatomic ions Polyatomic ions game
Polyatomic ions game Polyatomic ions sheet
Polyatomic ions sheet When adding a subscript to a polyatomic ion you should
When adding a subscript to a polyatomic ion you should Polyatomic ions quiz
Polyatomic ions quiz Polyatomic ions list with charges
Polyatomic ions list with charges