MRSA Infections Treatment Recommendations IDSA ATS Guidelines 2005



















- Slides: 23

MRSA Infections Treatment Recommendations

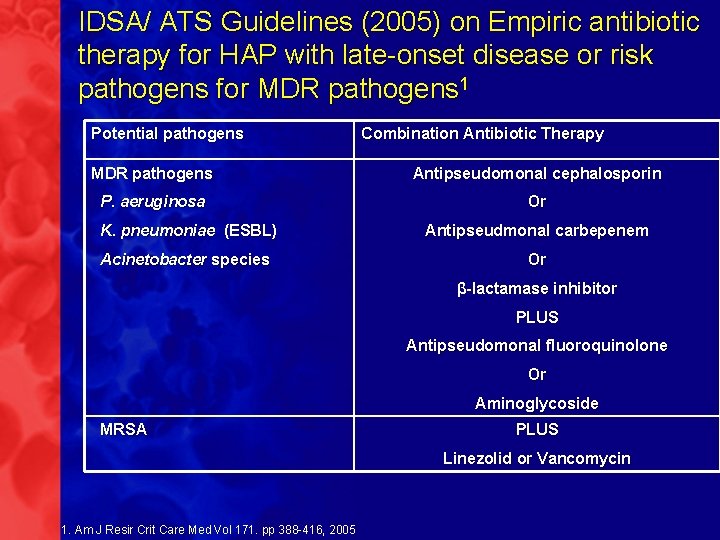
IDSA/ ATS Guidelines (2005) on Empiric antibiotic therapy for HAP with late-onset disease or risk pathogens for MDR pathogens 1 Potential pathogens Combination Antibiotic Therapy MDR pathogens Antipseudomonal cephalosporin P. aeruginosa Or K. pneumoniae (ESBL) Antipseudmonal carbepenem Acinetobacter species Or β-lactamase inhibitor PLUS Antipseudomonal fluoroquinolone Or Aminoglycoside MRSA PLUS Linezolid or Vancomycin 1. Am J Resir Crit Care Med Vol 171. pp 388 -416, 2005

MRSA guideline in the UK (2008) Published at JAC in Mar 2009

Use of Glycopeptides • “Vancomycin treatment failures occur with strains apparently susceptible in vitro. ” • “Infections with susceptible strains having MIC ≥ 1 mg are said to be more likely to fail on vancomycin therapy than those with susceptible strains having MICs <1 mg/L. ”

Use of Glycopeptides • “There is evidence that even high-dose vancomycin treatment does not improve the prognosis in renal patients, which may suggest that poor outcome is related to other biological factors. ” • “This might suggest that other treatment should be used for MRSA infections where the vancomycin MICs for the infecting strains are between 1 -4 mg/L and therefore vancomycin MICs should always be measured for MRSA treated with this drug”

Recommendation for MRSA RTI • Recommendation 12: • “The treatment of infections in patients with bronchiectasis or chronic suppurative lung disease without pneumonia, where MRSA is deemed significant, is unresolved. ” • “Linezolid may be used as it offers better lung tissue penetration [category 1 C]

Recommendation for MRSA RTI • “…PVL-positive strains of MRSA can cause severe necrotizing pneumonia in the community. In these circumstances, there is some in vitro evidence that staphylococcal toxin production can be suppressed by either clindamycin or linezolid” • “For community-onset and –acquired MRSA, we recommend that either linezolid, or if erythromycinsusceptible, clindamycin, should be included in therapy”

Recommendation for MRSA RTI • Recommendation 13: • “Glycopeptides or linezolid for pneumonia infections where MRSA is the aetiological agent. [category IA]”

Recommendation for MRSA Bacteraemia & Endocarditis • Recommendation 11: • “A minimum duration of 14 days of treatment with glycopeptides or linezolid fr uncomplicated bacteraemia” • “Longer treatment will be required in patients with, or at higher risk of, endocarditis, and transoesophageal echocardiographic assessment is important”

Recommendation for MRSA Bacteremia & Endocarditis • Recommendation 11 – “The manufacturer’s recommendation of a 4 week limit on linezolid treatment and adverse effects may limit use of this agent” – “Daptomycin could be considered as an alternative to glycopeptides [category II]”

Recommendation for MRSA SSTI • Impetigo and boils • Recommendation 3: – “Impetigo caused by MRSA should be treated with topical mupirocin or fusidic acid, where the isolate is susceptible [category II]” – “There is evidence for small boils that antibiotics are unnecessary and that drainage of lesions <5 cm diameter is adequate” • Recommendation 4 – “Antibiotic therapy is not generally required after incision and drainage of small abscesses without surrounding cellulitis. ”

Recommendation for MRSA SSTI • Cellulitis/ surgical site infections • Rec # 5 – non-hospitalized patients: – “Depending on susceptibility tests, doxycycline or clindamycin is recommendation unless the infections are considered severe and/ or carry a high risk of bacteremia or endocarditis [category 1 B]” – “With strains of MRSA resistant to doxycycline or clindamycin, glycopeptides or linezolid should be used. Co-trimoxazole could be considered, bearing in mind the hazards of sulphonamide allergy” – “Outpatient parenteral therapy with glycopeptide or daptomycin offers a cost-effective option in moderately severe infections where continuous IV therapy is deemed necessary [category 1 V]”

Recommendation for MRSA SSTI • Cellulitis/ surgical site infections • Rec # 5 – non-hospitalized patients: – “The possible superiority of linezolid in patients with proven complicated MRSA infection and in SSTIs, along with its availability as an oral agent with a high bioavailability, may also facilitate early hospital discharge and provides another cost effective alternative where appropriate. ” – “For suspected infections due to S. aureus or MRSA producing PVL, either linezolid or clindamycin should be considered since both have been shown to modify the expression of PVL in vitro. ”

Recommendation for MRSA SSTI • Cellulitis/ surgical site infections • Rec # 6 –hospitalized patients: – “Glycopeptides, linezolid or daptomycin should be considered for use in hospitalized patients with severe SSTIs and/or where the risk of bacteraemia is high. [category 1 B]” – “Linezolid may provide marginally greater effectiveness compared with glycopeptides in this patient population [category 1 B] – “If the infection is deemed polymicrobial and where MRSA is considered to be an important pathogen, monotherapy with tigecycline may be considered an alternative [category 1 B]”

Recommendation for MRSA SSTI • Cellulitis/ surgical site infections • Rec # 6 –hospitalized patients: – “For clinical treatment failure with glycopeptide monotherapy, we are unable to make a clear recommendation between adding a second antistaphylococcal agent to the glycopeptide, and switching to monotherapy with linezolid or daptomycin. ” – “A meta-analysis has suggested that linezolid may be superior to glycopeptides for Gram-positive infections in SSTIs and bacteraemia. ”

Recommendation for MRSA bone and joint infections • Rec#10 – “The treatment of MRSA bone and joint infection should be based on a multidisciplinary approach. ” – “Our previous recommendation to use parenteral glycopeptides with or without adjunctive agents such as rifampicin or sodium fusidate as initial parenteral therapy still stands. ” – “The Working Party considers that oral linezolid has a place in the follow-on treatment of bone and joint infections with MRSA after appropriate surgery, although this is not a licensed indication for the drug, but with weekly monitory of full blood count and platelets for signs of bone marrow suppression. ”

Recommendation for MRSA UTIs • Rec#9 – “For simple UTIs, an oral agent (nitrofurantoin, trimethoprim, co-trimoxazole or tetracycline) should be considered according to in vitro susceptibility [category II]” – “For complicated UTIs, glycopeptides or daptomycin should be considered. [category II]”

Recommendation for MRSA eye and CNS infections • Rec#14 – “There is insufficient evidence to make a specific recommendation in deep eye and CNS infection. ” – “Gentamicin, sodium fusidate or chloramphenicol may be used for superficial eye infections if the strain is susceptible [category 1 B]” – “Limited published data suggest that linezolid may be considered for the treatment of patients with meningeal or cerebral infections” – “Animal data suggest that daptomycin may have some advantages over vancomycin owing to its superior bactericidal activity. ”

Thank You

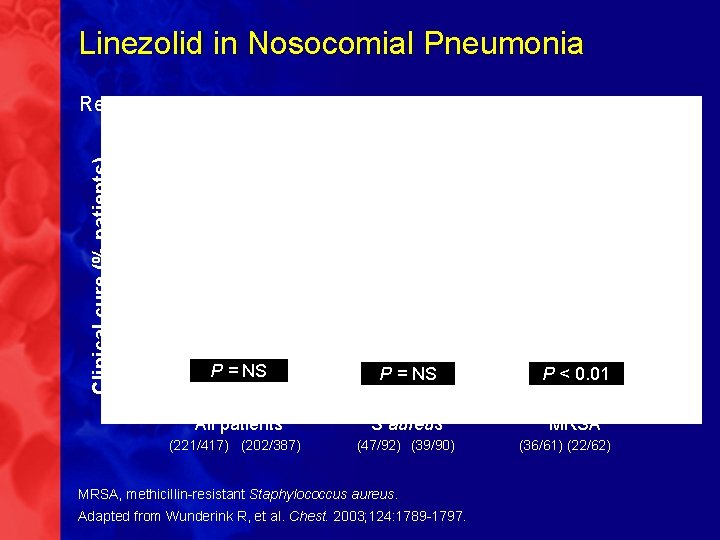
Linezolid in Nosocomial Pneumonia Clinical cure (% patients) Retrospective analysis of 2 randomized, double-blind studies P = NS P < 0. 01 All patients S aureus MRSA (221/417) (202/387) (47/92) (39/90) MRSA, methicillin-resistant Staphylococcus aureus. Adapted from Wunderink R, et al. Chest. 2003; 124: 1789 -1797. (36/61) (22/62)

Hospital-Acquired MRSA Pneumonia: Linezolid Versus Vancomycin Retrospective analysis of 2 randomized, double-blind studies Adapted from Wunderink R, et al. Chest. 2003; 124: 1789 -1797.

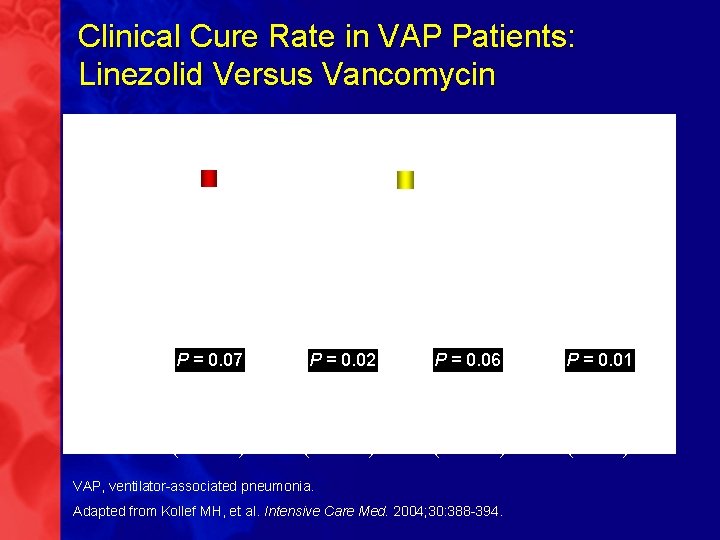
Clinical Cure Rate in VAP Patients: Linezolid Versus Vancomycin Retrospective analysis of 2 randomized, double-blind studies Vancomycin Linezolid P = 0. 07 P = 0. 02 P = 0. 06 P = 0. 01 (n = 434) (n = 214) (n = 179) (n = 70) VAP, ventilator-associated pneumonia. Adapted from Kollef MH, et al. Intensive Care Med. 2004; 30: 388 -394.

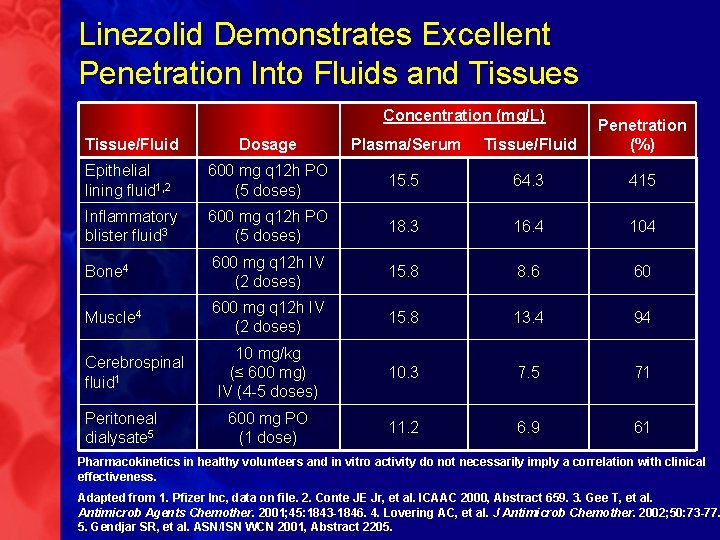
Linezolid Demonstrates Excellent Penetration Into Fluids and Tissues Concentration (mg/L) Tissue/Fluid Dosage Plasma/Serum Tissue/Fluid Penetration (%) Epithelial lining fluid 1, 2 600 mg q 12 h PO (5 doses) 15. 5 64. 3 415 Inflammatory blister fluid 3 600 mg q 12 h PO (5 doses) 18. 3 16. 4 104 Bone 4 600 mg q 12 h IV (2 doses) 15. 8 8. 6 60 Muscle 4 600 mg q 12 h IV (2 doses) 15. 8 13. 4 94 10 mg/kg (≤ 600 mg) IV (4 -5 doses) 10. 3 7. 5 71 600 mg PO (1 dose) 11. 2 6. 9 61 Cerebrospinal fluid 1 Peritoneal dialysate 5 Pharmacokinetics in healthy volunteers and in vitro activity do not necessarily imply a correlation with clinical effectiveness. Adapted from 1. Pfizer Inc, data on file. 2. Conte JE Jr, et al. ICAAC 2000, Abstract 659. 3. Gee T, et al. Antimicrob Agents Chemother. 2001; 45: 1843 -1846. 4. Lovering AC, et al. J Antimicrob Chemother. 2002; 50: 73 -77. 5. Gendjar SR, et al. ASN/ISN WCN 2001, Abstract 2205.
 Clabsi guidelines idsa
Clabsi guidelines idsa What is ncf 2005
What is ncf 2005 Ich treatment guidelines
Ich treatment guidelines Sbp treatment guidelines
Sbp treatment guidelines Hepatic encephalopathy staging
Hepatic encephalopathy staging Allergic rhinitis treatment guidelines
Allergic rhinitis treatment guidelines Transtubular potassium gradient
Transtubular potassium gradient Management of allergic rhinitis
Management of allergic rhinitis Official disability guidelines
Official disability guidelines Mrsa de centro
Mrsa de centro Mrsa wound
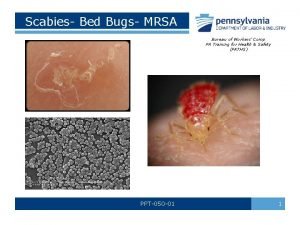
Mrsa wound Scabies
Scabies Ca mrsa
Ca mrsa Spannhake orthodontics
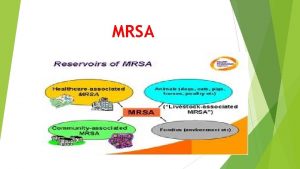
Spannhake orthodontics Mrsa
Mrsa Bakterija mrsa
Bakterija mrsa Mrsa
Mrsa Mrsa infekce
Mrsa infekce Mrsa
Mrsa Mrsa näyte fimlab
Mrsa näyte fimlab Mssa vs mrsa
Mssa vs mrsa Mrsa meddig fertőz
Mrsa meddig fertőz Mount royal staff association
Mount royal staff association What is mrsa in medical terms
What is mrsa in medical terms