Monitoring treatment success and failure in CML Andreas

![The International Scale (IS) for BCR-ABL LOCAL ASSAY International Scale 100% [IRIS baseline] 10% The International Scale (IS) for BCR-ABL LOCAL ASSAY International Scale 100% [IRIS baseline] 10%](https://slidetodoc.com/presentation_image_h/8eb51323b9d1cc7be388c25e8de54421/image-10.jpg)
![Definitions of Complete Molecular Response 100% [IRIS baseline] MR 4 (≥ 4 log reduction; Definitions of Complete Molecular Response 100% [IRIS baseline] MR 4 (≥ 4 log reduction;](https://slidetodoc.com/presentation_image_h/8eb51323b9d1cc7be388c25e8de54421/image-13.jpg)
- Slides: 21

Monitoring treatment success and failure in CML. Andreas Hochhaus Universitätsklinikum Jena, Germany

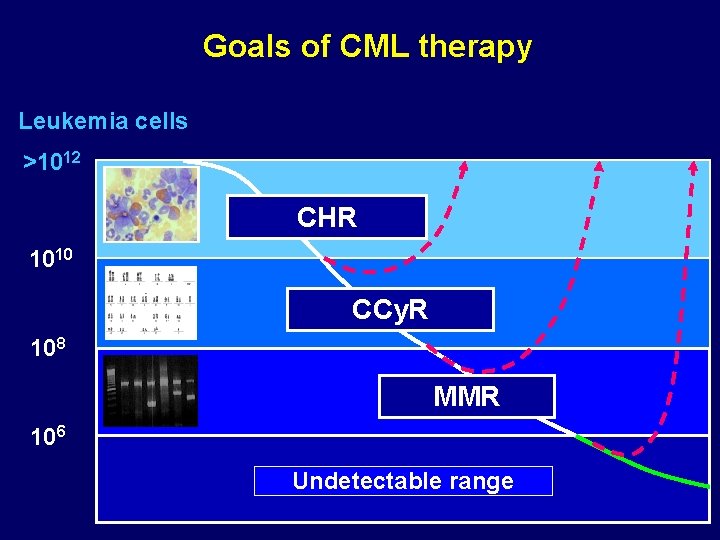
Goals of CML therapy Leukemia cells >1012 CHR 1010 CCy. R 108 MMR 106 Undetectable range

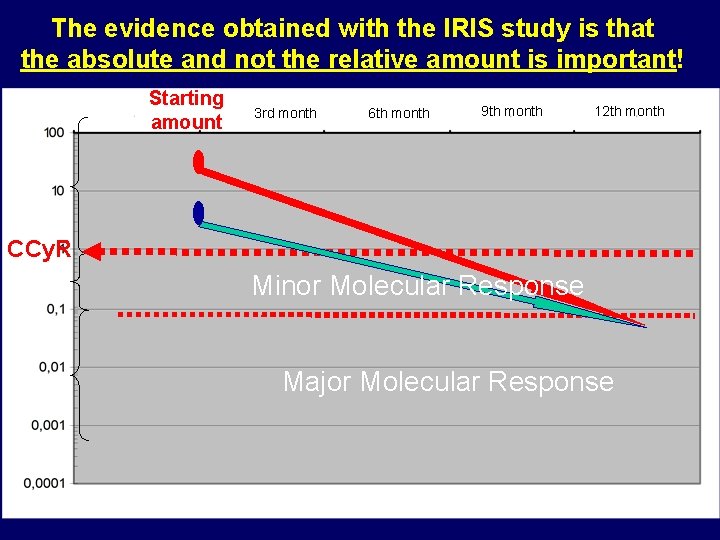
The evidence obtained with the IRIS study is that the absolute and not the relative amount is important! Starting amount 3 rd month 6 th month 9 th month 12 th month CCy. R Minor Molecular Response Major Molecular Response

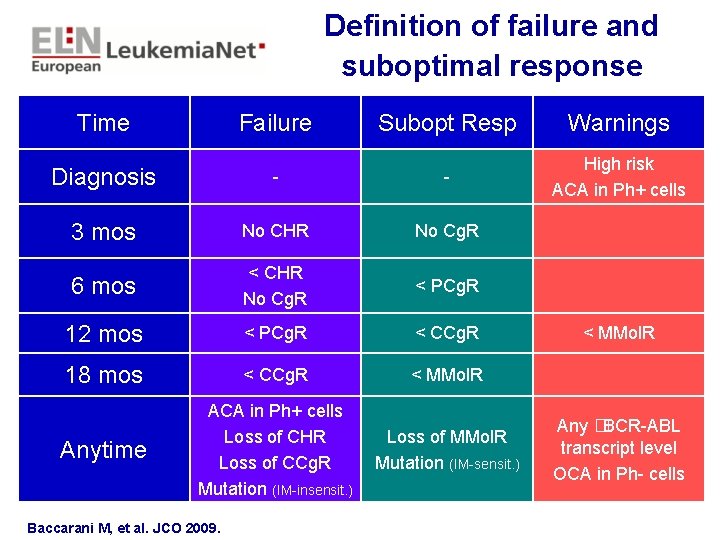
Definition of failure and suboptimal response Time Failure Subopt Resp Warnings Diagnosis - - High risk ACA in Ph+ cells 3 mos No CHR No Cg. R 6 mos < CHR No Cg. R < PCg. R 12 mos < PCg. R < CCg. R 18 mos < CCg. R < MMol. R Anytime ACA in Ph+ cells Loss of CHR Loss of CCg. R Mutation (IM-insensit. ) Loss of MMol. R Mutation (IM-sensit. ) Baccarani M, et al. JCO 2009. < MMol. R Any �BCR-ABL transcript level OCA in Ph- cells

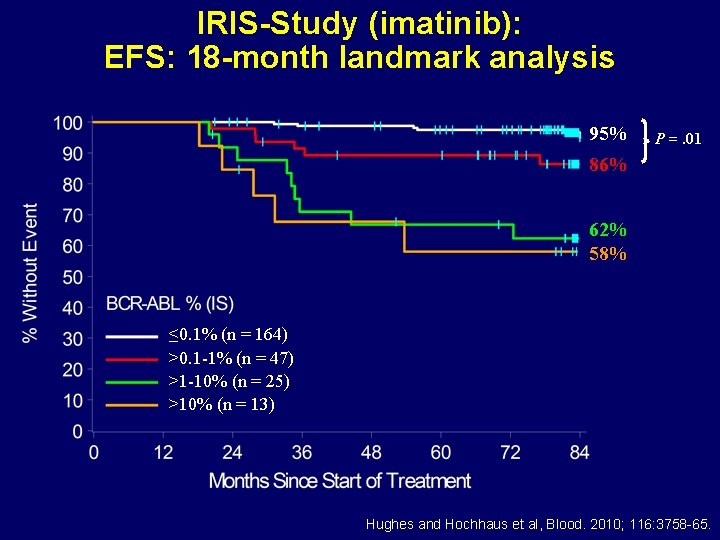
IRIS-Study (imatinib): EFS: 18 -month landmark analysis 95% P =. 01 86% 62% 58% ≤ 0. 1% (n = 164) >0. 1 -1% (n = 47) >1 -10% (n = 25) >10% (n = 13) Hughes and Hochhaus et al, Blood. 2010; 116: 3758 -65.

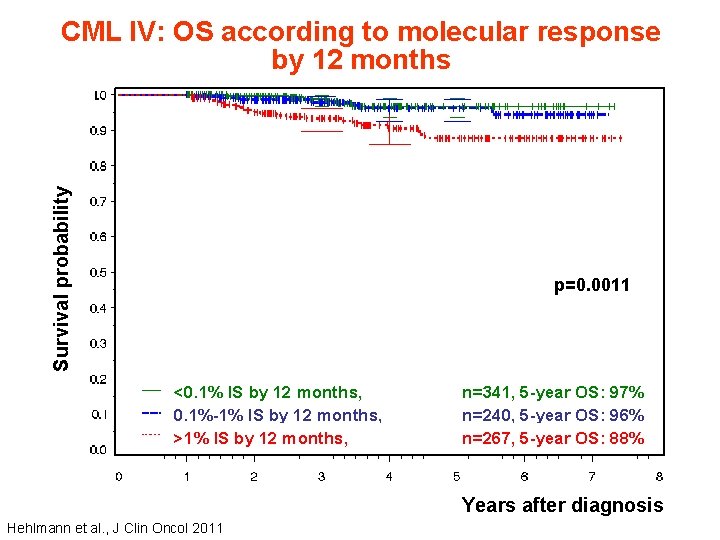
Survival probability CML IV: OS according to molecular response by 12 months p=0. 0011 <0. 1% IS by 12 months, 0. 1%-1% IS by 12 months, >1% IS by 12 months, n=341, 5 -year OS: 97% n=240, 5 -year OS: 96% n=267, 5 -year OS: 88% Years after diagnosis Hehlmann et al. , J Clin Oncol 2011

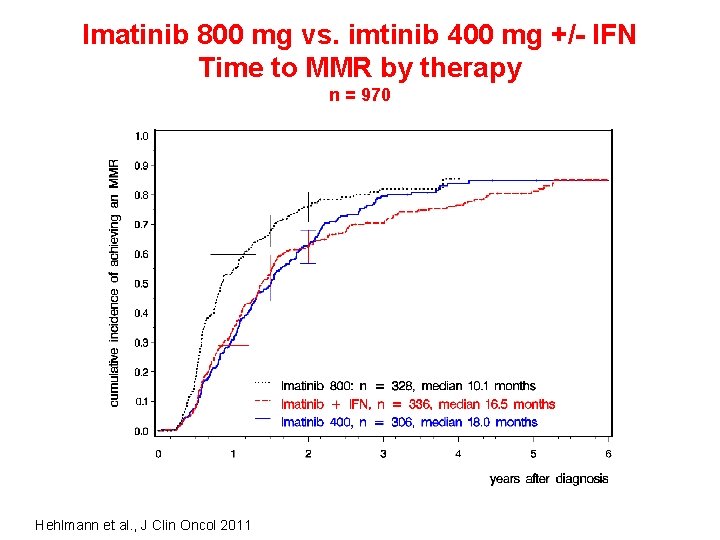
Imatinib 800 mg vs. imtinib 400 mg +/- IFN Time to MMR by therapy n = 970 Hehlmann et al. , J Clin Oncol 2011

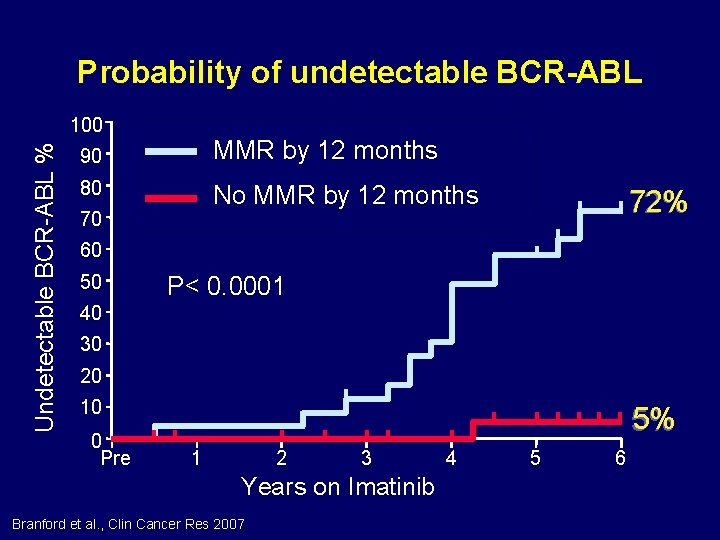
Probability of undetectable BCR-ABL Undetectable BCR-ABL % 100 90 MMR by 12 months 80 No MMR by 12 months 70 72% 60 50 P< 0. 0001 40 30 20 10 0 Pre 5% 1 2 3 Years on Imatinib Branford et al. , Clin Cancer Res 2007 4 5 6

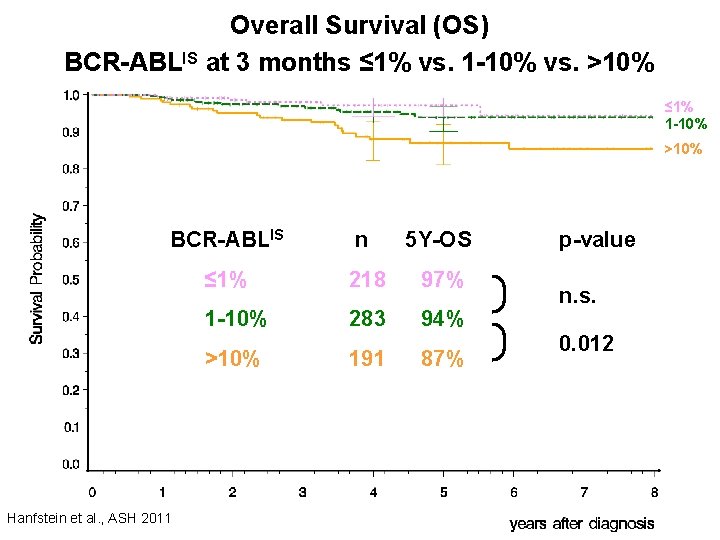
Overall Survival (OS) BCR-ABLIS at 3 months ≤ 1% vs. 1 -10% vs. >10% ≤ 1% 1 -10% >10% BCR-ABLIS n 5 Y-OS ≤ 1% 218 97% 1 -10% 283 94% >10% 191 87% Hanfstein et al. , ASH 2011 p-value n. s. 0. 012
![The International Scale IS for BCRABL LOCAL ASSAY International Scale 100 IRIS baseline 10 The International Scale (IS) for BCR-ABL LOCAL ASSAY International Scale 100% [IRIS baseline] 10%](https://slidetodoc.com/presentation_image_h/8eb51323b9d1cc7be388c25e8de54421/image-10.jpg)
The International Scale (IS) for BCR-ABL LOCAL ASSAY International Scale 100% [IRIS baseline] 10% Conversion factor/ Reference samples 1% 0. 01% 0. 001% Cross NCP. Best Pract Res Clin Haematol 2009; 22: 355 -365. [IRIS MMR; 3 log reduction]

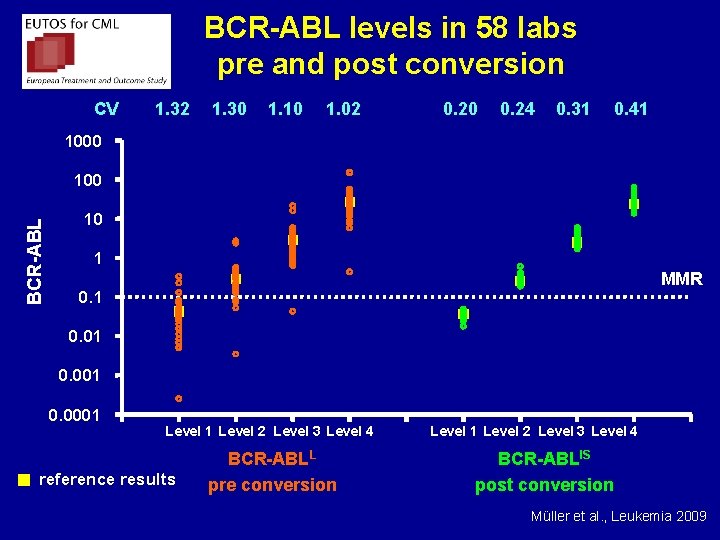
BCR-ABL levels in 58 labs pre and post conversion CV 1. 32 1. 30 1. 10 1. 02 0. 20 0. 24 0. 31 0. 41 1000 BCR-ABL 100 10 1 MMR 0. 1 0. 001 0. 0001 Level 2 Level 3 Level 4 reference results BCR-ABLL pre conversion Level 1 Level 2 Level 3 Level 4 BCR-ABLIS post conversion Müller et al. , Leukemia 2009

Monitoring: ELN Recommendations Diagnosis § § BM aspiration cytology (+biopsy) BM cytogenetics PB Multiplex PCR (Type of BCR-ABL transcript) (PB FISH) Follow up § § § BM cytogenetics 3 monthly until CCy. R q. RT-PCR 3 -6 monthly (interphase FISH to confirm CCy. R, if PCR is not available) Systematic monitoring is the best motivation for adherence. Baccarani et al. , J Clin Oncol. 2009
![Definitions of Complete Molecular Response 100 IRIS baseline MR 4 4 log reduction Definitions of Complete Molecular Response 100% [IRIS baseline] MR 4 (≥ 4 log reduction;](https://slidetodoc.com/presentation_image_h/8eb51323b9d1cc7be388c25e8de54421/image-13.jpg)
Definitions of Complete Molecular Response 100% [IRIS baseline] MR 4 (≥ 4 log reduction; ≤ 0. 01%IS) 10% 1% MR 4. 5 (≥ 4. 5 log reduction; ≤ 0. 0032%IS) 0. 1% [IRIS MMR] 0. 01% MR 5 (≥ 5 log reduction; ≤ 0. 001%IS) 0. 001% BCR-ABL undetectable log reduction = reduction from IRIS baseline, not individual pretreatment levels International Scale

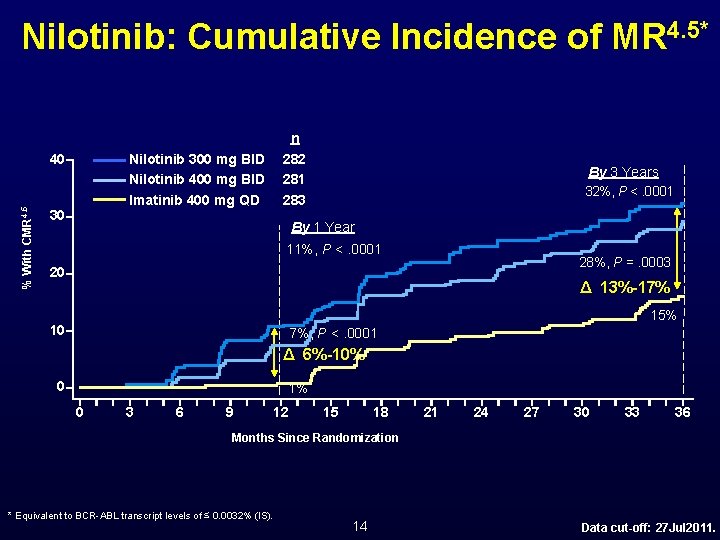
Nilotinib: Cumulative Incidence of MR 4. 5* % With CMR 4. 5 40 n 282 281 283 Nilotinib 300 mg BID Nilotinib 400 mg BID Imatinib 400 mg QD 30 By 3 Years 32%, P <. 0001 By 1 Year 11%, P <. 0001 28%, P =. 0003 20 Δ 13%-17% 15% 10 7%, P <. 0001 Δ 6%-10% 0 1% 0 3 6 9 12 15 18 21 24 27 30 33 36 Months Since Randomization * Equivalent to BCR-ABL transcript levels of ≤ 0. 0032% (IS). 14 Data cut-off: 27 Jul 2011.

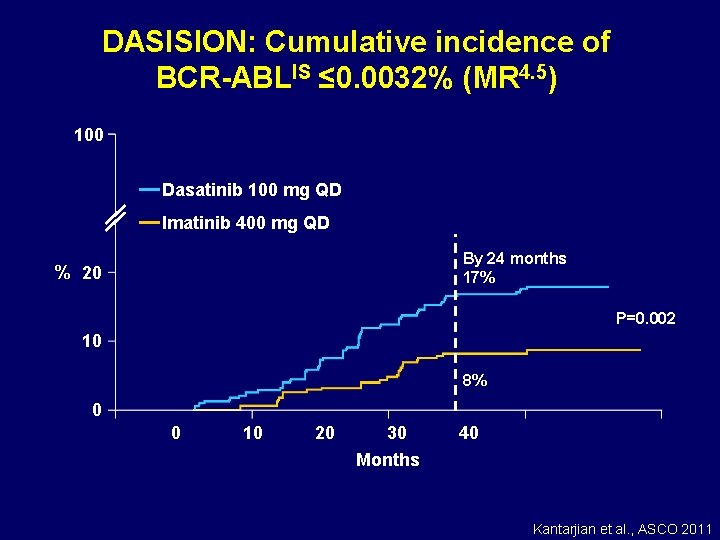
DASISION: Cumulative incidence of BCR-ABLIS ≤ 0. 0032% (MR 4. 5) 100 Dasatinib 100 mg QD Imatinib 400 mg QD By 24 months 17% % 20 P=0. 002 10 8% 0 0 10 20 30 Months 40 Kantarjian et al. , ASCO 2011

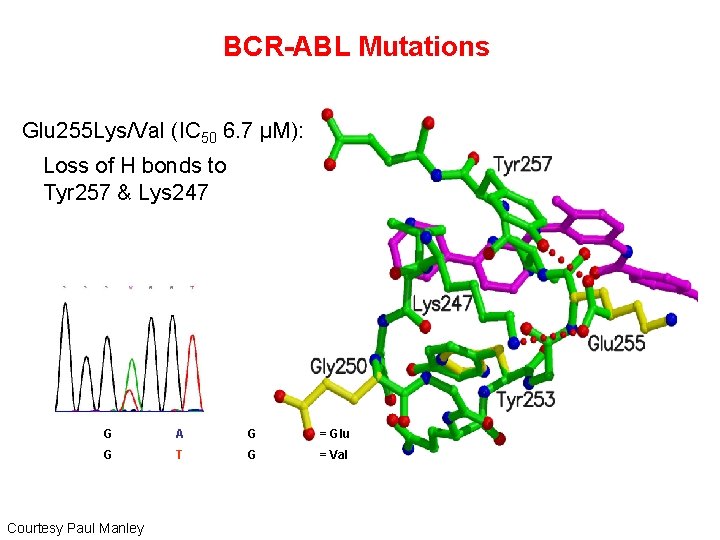
BCR-ABL Mutations Glu 255 Lys/Val (IC 50 6. 7 μM): § Loss of H bonds to Tyr 257 & Lys 247 G A G = Glu G T G = Val Courtesy Paul Manley

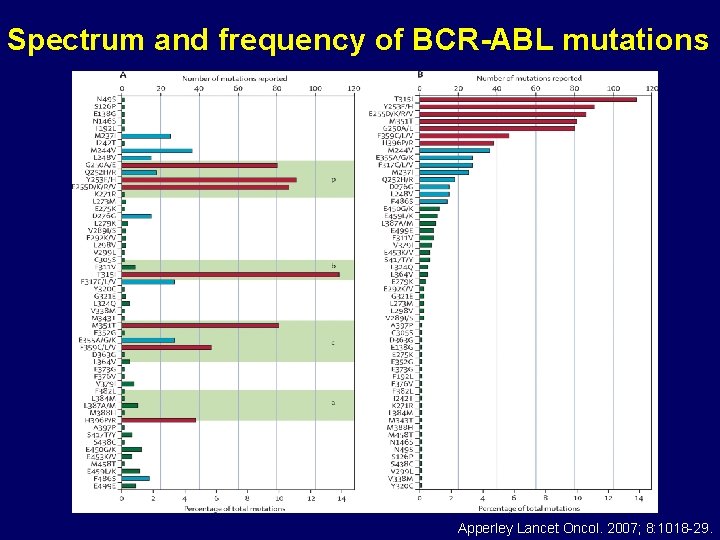
Spectrum and frequency of BCR-ABL mutations Apperley Lancet Oncol. 2007; 8: 1018 -29.

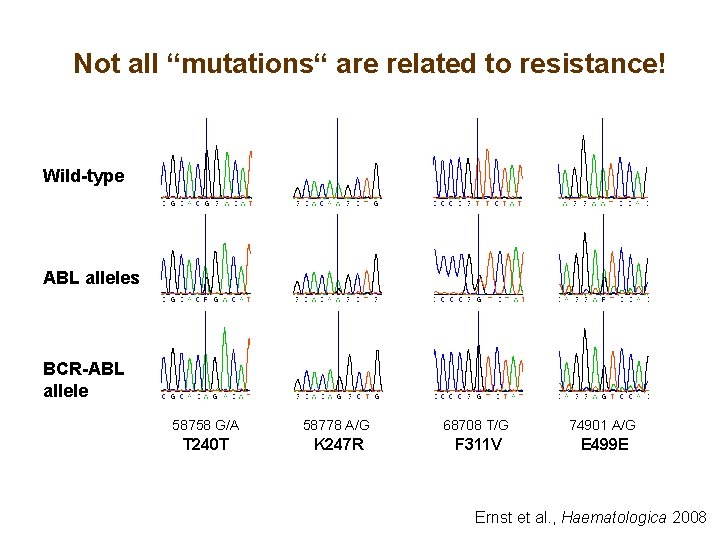
Not all “mutations“ are related to resistance! Wild-type ABL alleles BCR-ABL allele 58758 G/A 58778 A/G 68708 T/G 74901 A/G T 240 T K 247 R F 311 V E 499 E Ernst et al. , Haematologica 2008

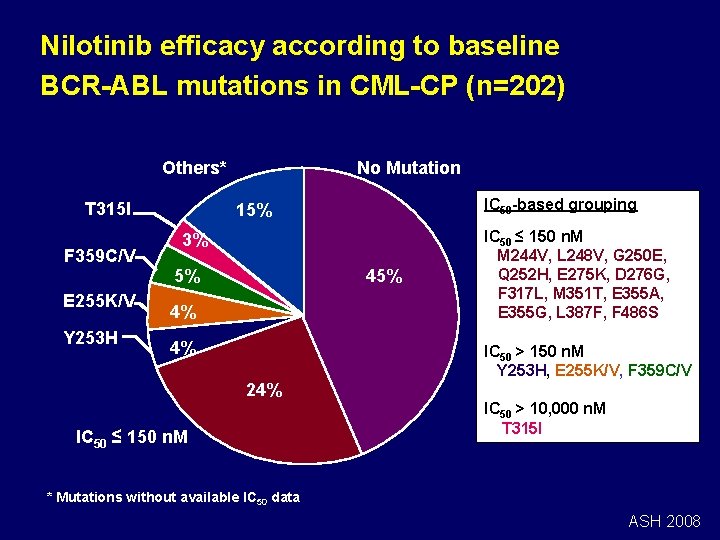
Nilotinib efficacy according to baseline BCR-ABL mutations in CML-CP (n=202) No Mutation Others* T 315 I F 359 C/V E 255 K/V Y 253 H IC 50 -based grouping 15% 3% 5% 4% 4% IC 50 ≤ 150 n. M M 244 V, L 248 V, G 250 E, Q 252 H, E 275 K, D 276 G, F 317 L, M 351 T, E 355 A, E 355 G, L 387 F, F 486 S IC 50 > 150 n. M Y 253 H, E 255 K/V, F 359 C/V 24% IC 50 ≤ 150 n. M IC 50 > 10, 000 n. M T 315 I * Mutations without available IC 50 data ASH 2008

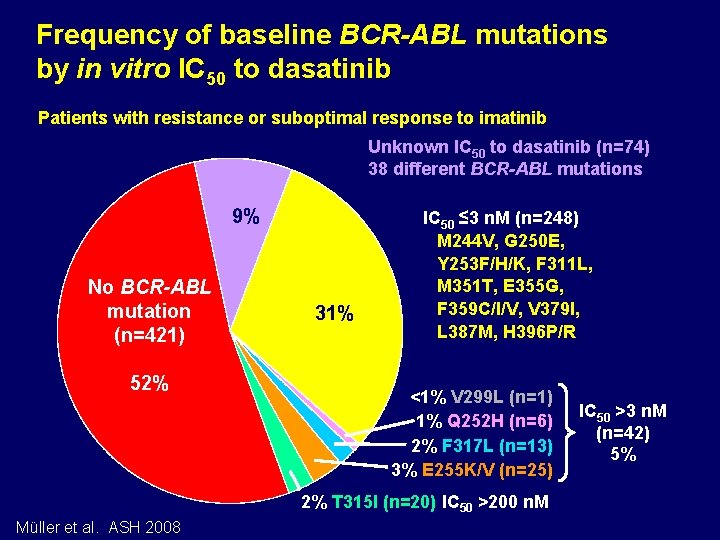
Frequency of baseline BCR-ABL mutations by in vitro IC 50 to dasatinib Patients with resistance or suboptimal response to imatinib Unknown IC 50 to dasatinib (n=74) 38 different BCR-ABL mutations 9% No BCR-ABL mutation (n=421) 52% 31% IC 50 ≤ 3 n. M (n=248) M 244 V, G 250 E, Y 253 F/H/K, F 311 L, M 351 T, E 355 G, F 359 C/I/V, V 379 I, L 387 M, H 396 P/R <1% V 299 L (n=1) 1% Q 252 H (n=6) 2% F 317 L (n=13) 3% E 255 K/V (n=25) 2% T 315 I (n=20) IC 50 >200 n. M Müller et al. ASH 2008 IC 50 >3 n. M (n=42) 5%

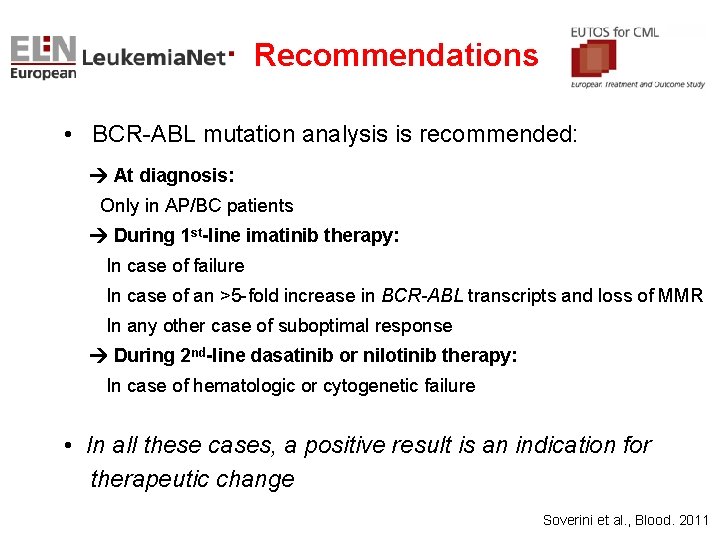
Recommendations • BCR-ABL mutation analysis is recommended: At diagnosis: Only in AP/BC patients During 1 st-line imatinib therapy: In case of failure In case of an >5 -fold increase in BCR-ABL transcripts and loss of MMR In any other case of suboptimal response During 2 nd-line dasatinib or nilotinib therapy: In case of hematologic or cytogenetic failure • In all these cases, a positive result is an indication for therapeutic change Soverini et al. , Blood. 2011
 Chronic myeloid leukemia treatment
Chronic myeloid leukemia treatment Standardised cml monitoring
Standardised cml monitoring Ventricular escape rhythm
Ventricular escape rhythm Failure to pace
Failure to pace Cup and cone fracture ductile or brittle
Cup and cone fracture ductile or brittle Factors of project success and failure
Factors of project success and failure People in service marketing
People in service marketing Cycle of failure mediocrity and success
Cycle of failure mediocrity and success Was dunkirk a success or failure
Was dunkirk a success or failure Cml
Cml Cml 100
Cml 100 Equation of capital market line
Equation of capital market line Diagnosi cml
Diagnosi cml Procesadores cml microcircuits
Procesadores cml microcircuits Assumption of capm
Assumption of capm Que es numero natural
Que es numero natural Obm cml
Obm cml Cml awareness day
Cml awareness day Rumus security market line
Rumus security market line Cml
Cml Cml
Cml 20115n-cml
20115n-cml