METRICS Measurement What is measurement any standard of






















- Slides: 30

***METRICS***

Measurement • What is measurement? – any standard of comparison, estimation, or judgment

Why do we measure things? – To give information – To compare things quantitatively (if a person had never seen or heard of these animals before, measurements would help that person compare them) – Length of a crocodile compared to a gecko – Height of a Basketball player, compared to horse jockey – Weight of an elephant, compared to mouse

• What kinds of measurements can you take? – Length – Volume – Mass – Temperature

U. S. A • What kind of scale does the U. S. use? – U. S. Customary scale • Including inches, feet, miles

The World & The Scientific Community – Uses the SI Systeme Internationale d’Unites (French), or Metric System – As early as the 17 th century French scientist found it illogical to have so many measurement systems so they developed one universal system. • It is easy to use because it is in multiples of 10 • Today all countries except the United States, Burma, and Liberia use the metric system

• Why is it important to use only one scale of measurement in science? – Scientists need to be able to share and compare their work with other scientists easily. – It makes it less confusing!

Length • Basic unit of measure = meter • What would you measure in meters? – 1. How far you throw a softball – 2. A person’s height – 3. Measure height of a room’s wall

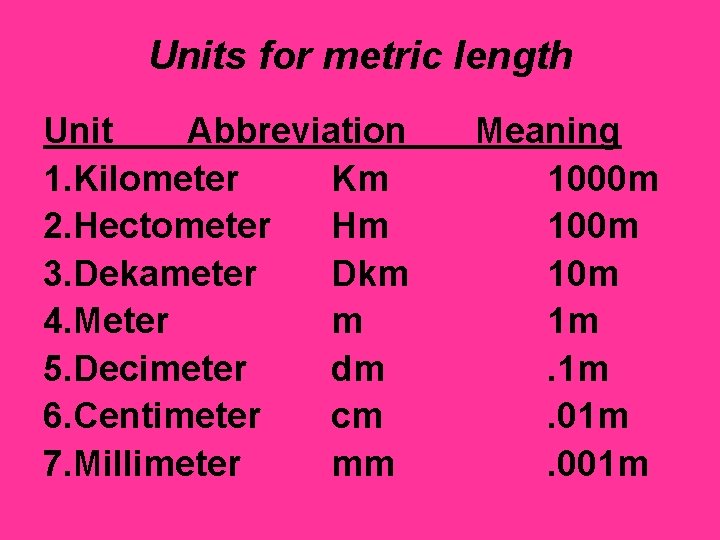
Units for metric length Unit Abbreviation 1. Kilometer Km 2. Hectometer Hm 3. Dekameter Dkm 4. Meter m 5. Decimeter dm 6. Centimeter cm 7. Millimeter mm Meaning 1000 m 10 m 1 m. 01 m. 001 m

Multiples of Ten 10 Hm 10 Dkm 10 dm 10 cm 10 mm = 1 Km = 1 Hm = 1 Dkm =1 m = 1 dm = 1 cm

King Henry Died Making Dead Cows Moo

King Kilometer Henry Hectometer Died Dekameter Making Meter Dead Decimeter Cows Centimeter Moo Millimeter

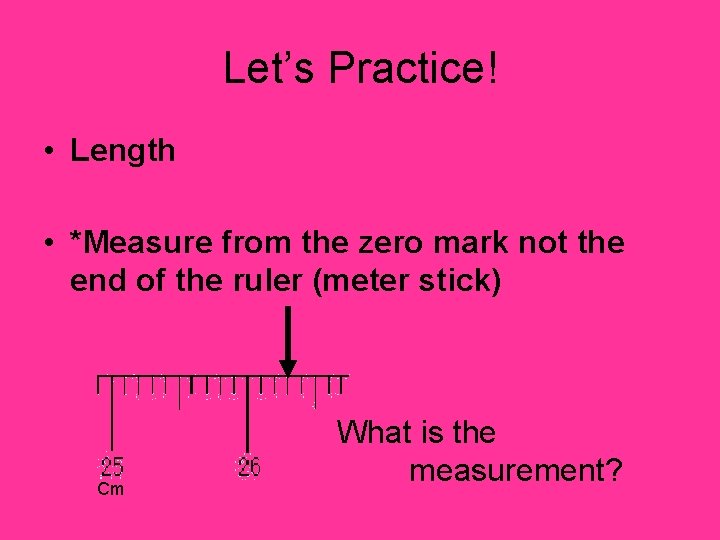
Let’s Practice! • Length • *Measure from the zero mark not the end of the ruler (meter stick) Cm What is the measurement?

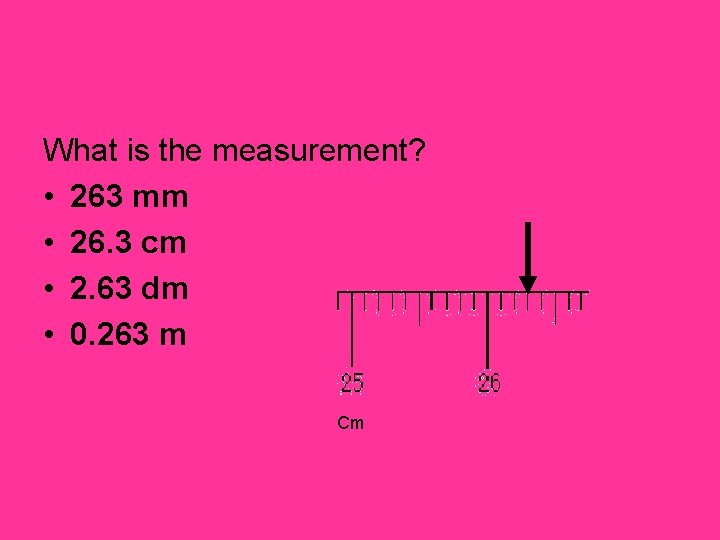
What is the measurement? • 263 mm • 26. 3 cm • 2. 63 dm • 0. 263 m Cm

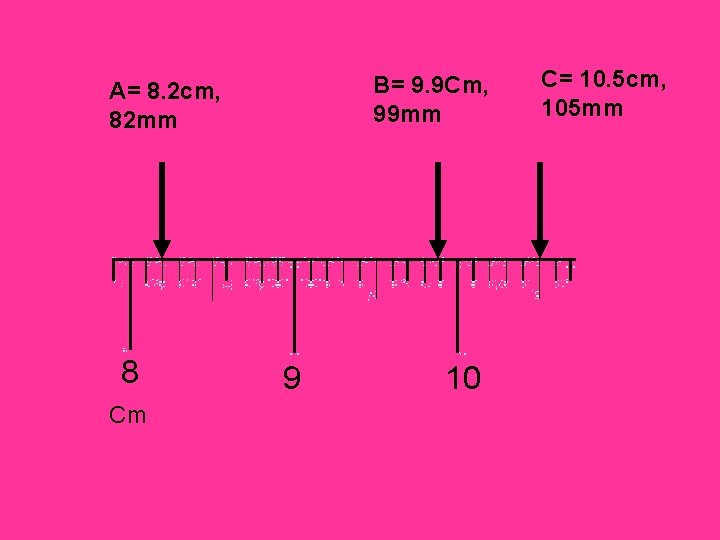
One More, On Your Own What are these measurements in centimeters, and millimeters? A 8 Cm B 9 C 10

B= 9. 9 Cm, 99 mm A= 8. 2 cm, 82 mm 8 Cm 9 10 C= 10. 5 cm, 105 mm

Mass, Temperature, and Time

Mass Basic unit of mass: = gram (g)

Measuring Mass Using a triple beam balance 1. Place object on the pan 2. Move rider on the middle beam notch by notch until the horizontal pointer drops below zero. Move the rider back one notch 3. Move the rider on the back beam notch by notch until the pointer again drops below zero. Move the rider back one notch. 4. Slowly slide the rider along the front beam until the pointer stops at the zero point. 5. The mass of the object is equal to the sum of the readings on the three beams.

Mass vs. Weight • Mass – the amount of matter an object contains (the amount of stuff) IT DOES NOT CHANGE • Weight- force of gravity on an object – Can vary depending on location of object. • Example: – Your mass is the same on the moon and on the earth. – Your weight is different on the moon as compared to the earth. • You weigh less on the moon because the force of gravity is less on the moon.

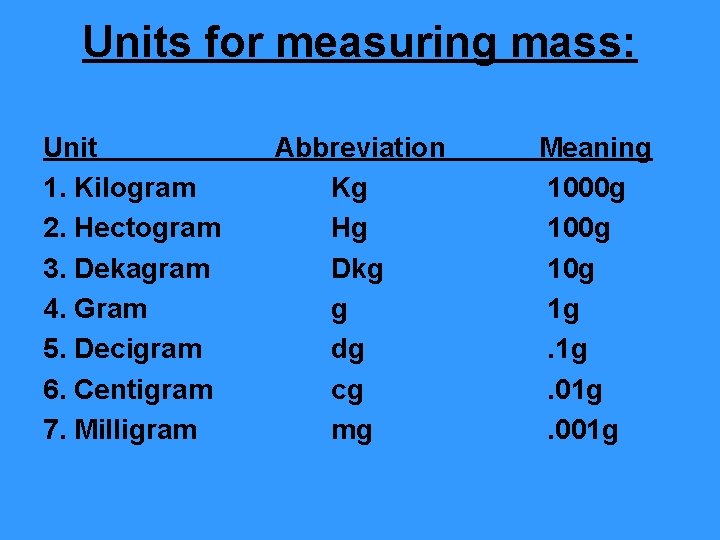
Units for measuring mass: Unit 1. Kilogram 2. Hectogram 3. Dekagram 4. Gram 5. Decigram 6. Centigram 7. Milligram Abbreviation Kg Hg Dkg g dg cg mg Meaning 1000 g 10 g 1 g. 01 g. 001 g

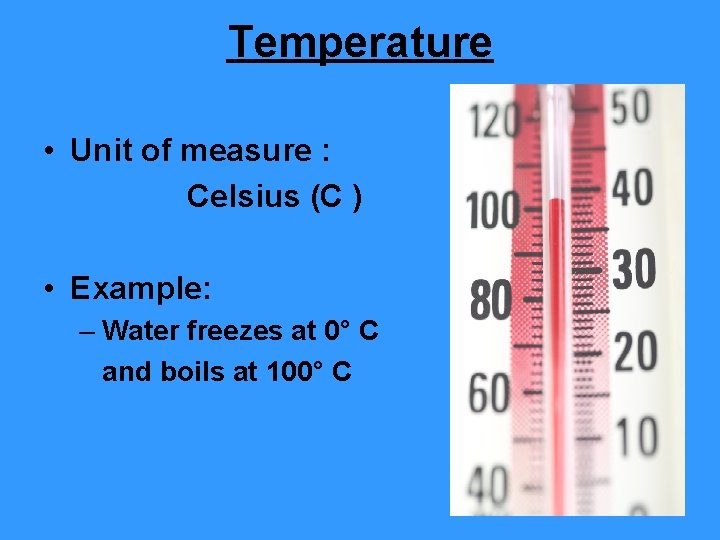
Temperature • Unit of measure : Celsius (C ) • Example: – Water freezes at 0° C and boils at 100° C

Time • Time is simply distinguishing which of two events is earlier and which later • Time is measured in different increments • Years • Months • Days • Hours • Minutes • Seconds

Volume and Density

Volume - The amount of space an object takes up.

Measuring Volume • Liquids – Unit of measure = Liters (L) or (ml) – Use a graduated cylinder – Measure from the bottom of the meniscus • Meniscus –the curved surface of a liquid 20 15 10

Measuring Volume • Regular solids – Unit of measure = cubic centimeter (cm 3) – Volume= length x width x height Or L x W x H= Volume • Example – Volume = 20 cm x 6 cm x 25 cm = 3000 cm 3

Irregular Solids • Unit of measure= centimeters cubed (cm 3). • BUT! Measured using ml – ml and cm 3 are equivalent units! • Example: – Volume of a rock using a graduated cylinder

Density • Density: How much mass in a given volume (How much “stuff” in a certain amount of “space”) • Formula: Density = mass volume D=M V

• Example: – A box has a mass of 400 g and a volume of 15 cm 3. What is the density of the box? • Example: – Bowling ball vs. Beach ball • Same volume different mass • Which item is more dense?
 Software metrics and software metrology
Software metrics and software metrology Isms metrics
Isms metrics Any to any connectivity
Any to any connectivity Seknder
Seknder No, there aren’t.
No, there aren’t. Standard measurement system
Standard measurement system Standar tegangan sesuai satuan internasional
Standar tegangan sesuai satuan internasional Peruntukan masa kssm 2020 menengah rendah
Peruntukan masa kssm 2020 menengah rendah Standard error in the mean
Standard error in the mean Differences between home language and standard language
Differences between home language and standard language Standard costs meaning
Standard costs meaning Fox metrics
Fox metrics What are the design metrics of embedded systems
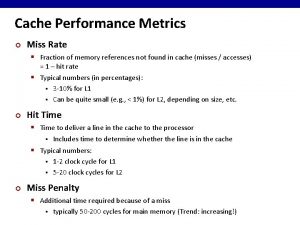
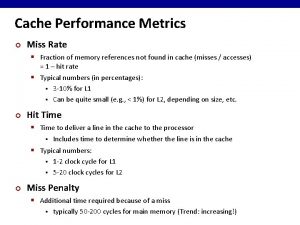
What are the design metrics of embedded systems Cache performance metrics
Cache performance metrics Sally elatta presents on business agility
Sally elatta presents on business agility Information flow metrics
Information flow metrics Engineering performance metrics
Engineering performance metrics Geneva monitoring agent
Geneva monitoring agent Training and development metrics
Training and development metrics Treasury kpi metrics
Treasury kpi metrics Sustainability metrics software
Sustainability metrics software Cache performance metrics
Cache performance metrics Facility related metrics
Facility related metrics Microconversion prediction
Microconversion prediction Erg metrics
Erg metrics How to measure digital customer experience
How to measure digital customer experience Boehm's top 10 principles
Boehm's top 10 principles Weighted methods per class
Weighted methods per class Snowflake metrics
Snowflake metrics Cloud computing business continuity planning
Cloud computing business continuity planning Project metrics in software engineering
Project metrics in software engineering