MENA 3100 1 st lecture General information what




- Slides: 25

MENA 3100 1 st lecture General information, what to learn and some repetition of crystallography 19/1 -10 MENA 3100

Student contact information Name 19/1 -10 E-mail Phone MENA 3100

Who is involved? • Anette E. Gunnæs: eleonora(at)fys. uio. no, 91514080 (General, TEM, ED) • • • Johan Taftø: johan. tafto(at)fys. uio. no (waves optics, TEM, EELS) Ole Bjørn Karlsen: obkarlsen(at)fys. uio. no (OM, XRD) Sissel Jørgensen: sissel. jorgensen(at)kjemi. uio. no (EDS, XPS) Spyros Diplas: spyros. diplas(at)smn. uio. no (XPS) Klaus Magnus Johansen: k. m. h. johansen(at)smn. uio. no (SIMS) Terje Finnstad: terje. finnstad(at)fys. uio. no (SPM) Oddvar Dyrlie: oddvar. dyrlie(at)kjemi. uio. no (SPM) Magnus Sørby: magnus. sorby(at)IFE. no (ND) Geir Helgesen: geir. helgesen(at)IFE. no (ND) Truls Norby: (SEM) 19/1 -10 MENA 3100

General information • Lectures – Based on D. Brandon and W. D. Kaplan "Microstructural characterization of materials". Second edition, published by Wiley, 2008. – The curriculum is made up of the whole book, except these chapters: 3. 4. 3, 3. 4. 4, 3. 4. 6, 7. 3 and 9. – Some parts of the Brandon and Kaplan book will be regarded as self study material – Lecture notes that are made available on the course webpage are also considered to be part of the curriculum. • Project work – – • Projects will be announced by the end of January/beginning of February Three students will work together, rank projects with 1 st-3 rd priority Written report, oral presentation and individual examination Counts 40 % of final grade Laboratories – Four groups: A, B, C, D (4 -5 students in one group) – Individual reports – All reports have to be evaluated and found ok before final written exam 19/1 -10 MENA 3100

Laboratory groups A C (Tue. 14. 15 - ) B (Tue. 16. 15 - ) (Wed. 10. 15 - ) D (Wed. 12. 15 - ) A trip to IFE, Kjeller has been scheduled to Wednesday 17 th of February! 19/1 -10 MENA 3100

What to learn about • Imaging/microscopy • – Optical – Electron Spectroscopy – EDS • X-rays – EELS • SEM • STEM • TEM • Electrons – XPS, AES – Scanning probe • Electrons (surface) • AFM • STM – SIMS • Ions Different imaging modes. • Diffraction – X-rays – Electrons Mapping of elements or chemical states of elements. • – – • ED in TEM and EBSD in SEM – Neutrons The same basic theory for all waves. 19/1 -10 Sample preparation MENA 3100 Mechanical grinding/polishing Chemical polishing/etching Ion bombardment Crunching etc……

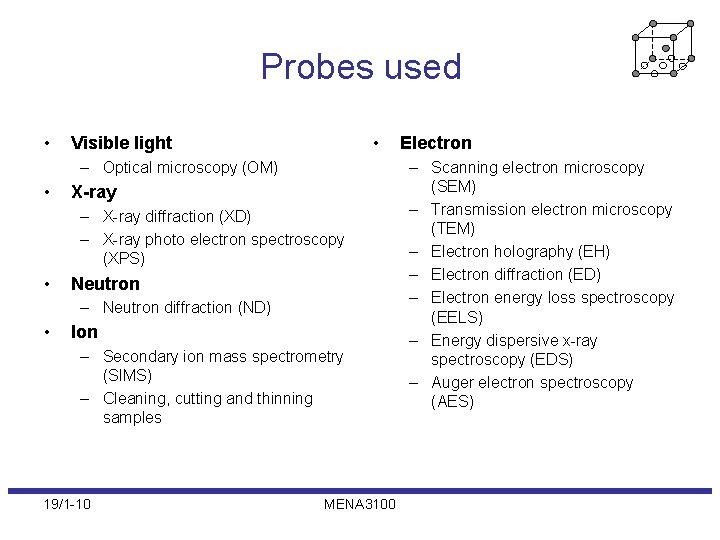
Probes used • • Visible light – Optical microscopy (OM) • X-ray – X-ray diffraction (XD) – X-ray photo electron spectroscopy (XPS) • Neutron – Neutron diffraction (ND) • Ion – Secondary ion mass spectrometry (SIMS) – Cleaning, cutting and thinning samples 19/1 -10 MENA 3100 Electron – Scanning electron microscopy (SEM) – Transmission electron microscopy (TEM) – Electron holography (EH) – Electron diffraction (ED) – Electron energy loss spectroscopy (EELS) – Energy dispersive x-ray spectroscopy (EDS) – Auger electron spectroscopy (AES)

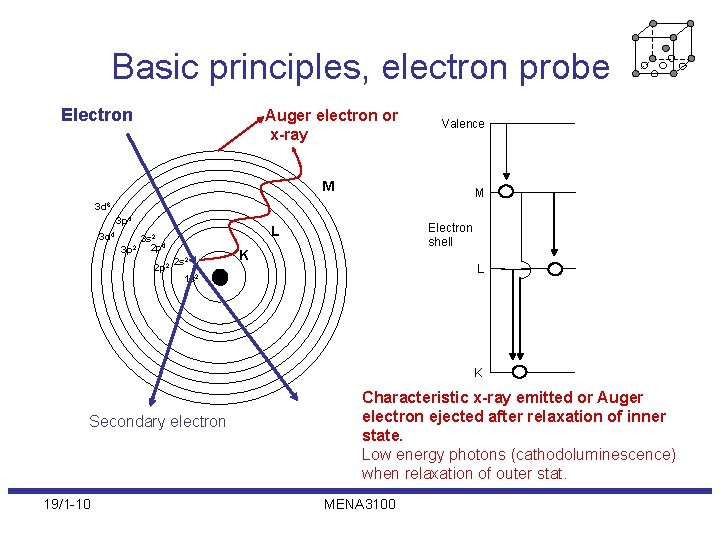
Basic principles, electron probe Electron Auger electron or x-ray Valence M M 3 d 6 3 p 4 3 d 4 2 p 2 Electron shell L 3 s 2 2 2 p 4 3 p 2 s 2 K L 1 s 2 K Secondary electron 19/1 -10 Characteristic x-ray emitted or Auger electron ejected after relaxation of inner state. Low energy photons (cathodoluminescence) when relaxation of outer stat. MENA 3100

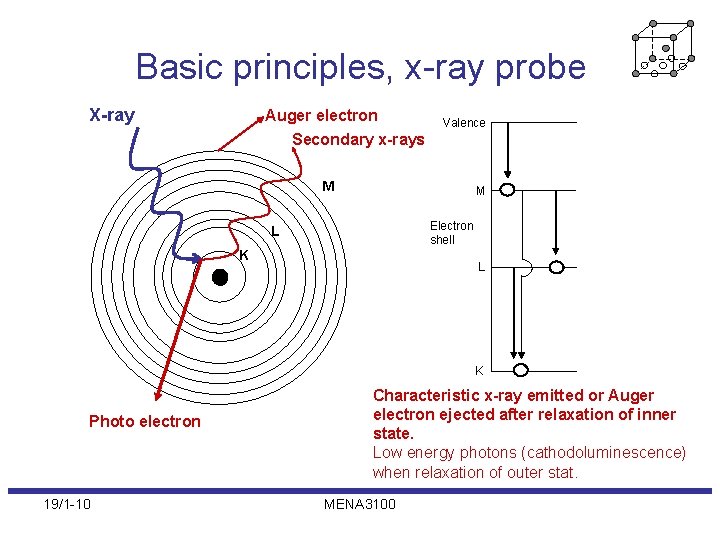
Basic principles, x-ray probe X-ray Auger electron Valence Secondary x-rays M M Electron shell L K Photo electron 19/1 -10 Characteristic x-ray emitted or Auger electron ejected after relaxation of inner state. Low energy photons (cathodoluminescence) when relaxation of outer stat. MENA 3100

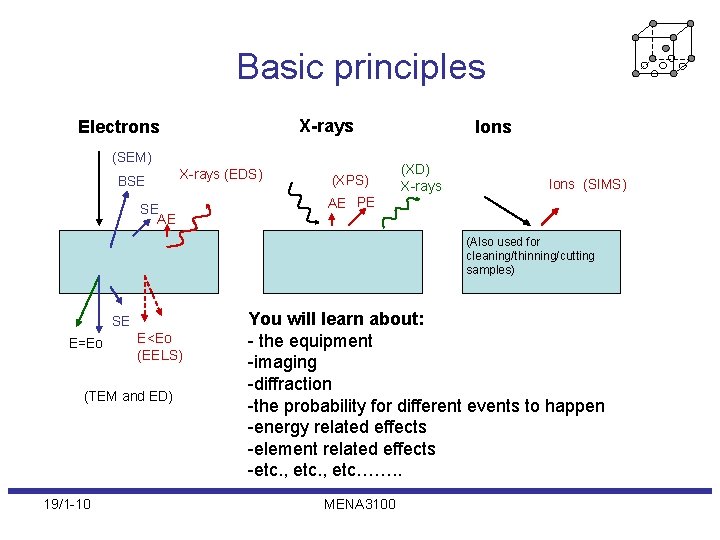
Basic principles X-rays Electrons (SEM) BSE X-rays (EDS) SE AE (XPS) Ions (XD) X-rays Ions (SIMS) AE PE (Also used for cleaning/thinning/cutting samples) SE E=Eo E<Eo (EELS) (TEM and ED) 19/1 -10 You will learn about: - the equipment -imaging -diffraction -the probability for different events to happen -energy related effects -element related effects -etc. , etc……. . MENA 3100

Introduction to crystallography We divide materials into two categories: – Amorphous materials • The atoms are ”randomly” distributed in space • Not quite true, there is short range order • Examples: glass, polystyrene (isopor) – Crystalline materials • The atoms are perfectly ordered • Short range and long range order • Deviations from the perfect order are important 19/1 -10 MENA 3100

Introduction to crystallography 19/1 -10 MENA 3100

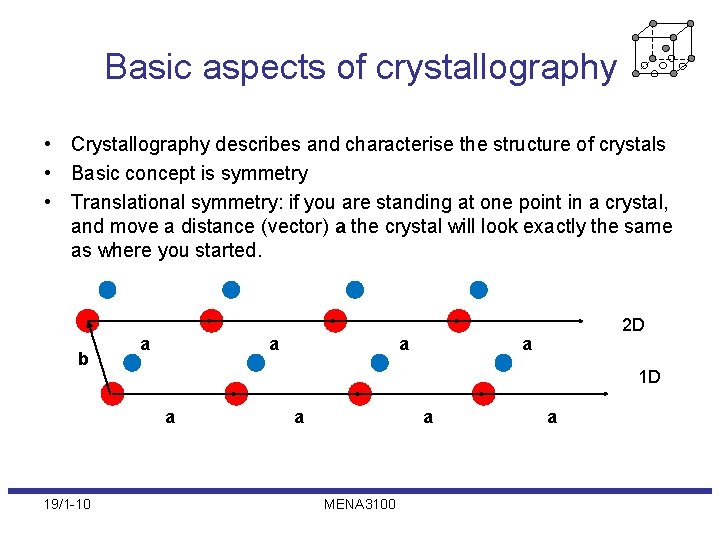
Basic aspects of crystallography • Crystallography describes and characterise the structure of crystals • Basic concept is symmetry • Translational symmetry: if you are standing at one point in a crystal, and move a distance (vector) a the crystal will look exactly the same as where you started. b a a a 1 D a 19/1 -10 a 2 D a a MENA 3100 a

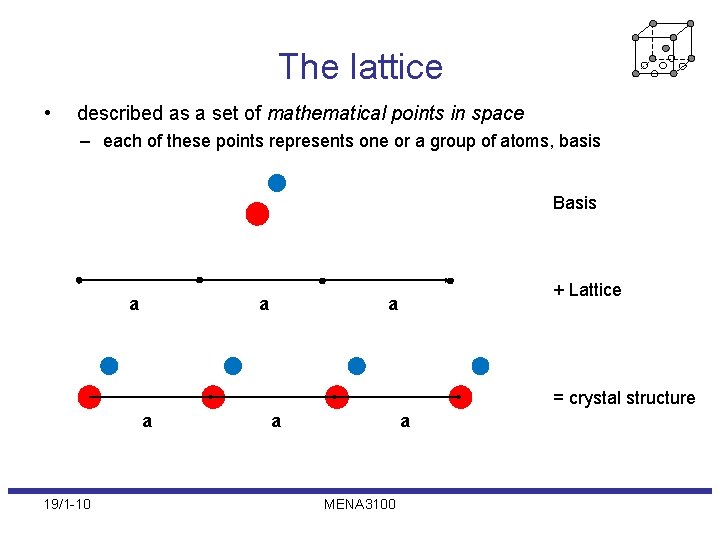
The lattice • described as a set of mathematical points in space – each of these points represents one or a group of atoms, basis Basis a a + Lattice a = crystal structure a 19/1 -10 a a MENA 3100

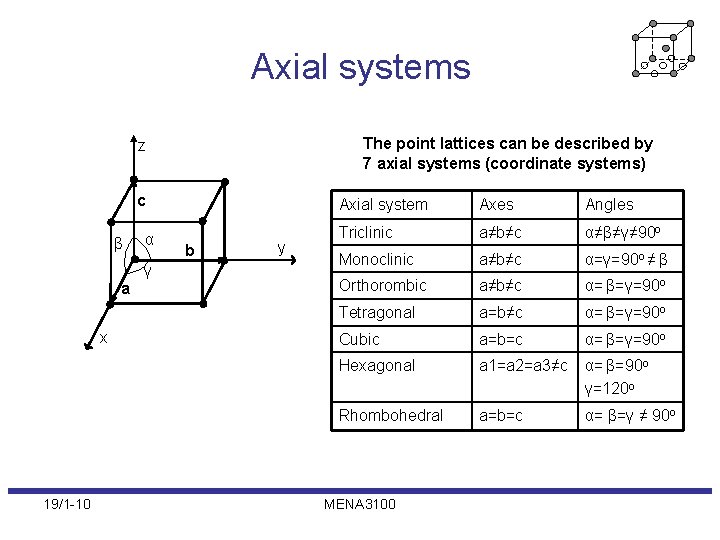
Axial systems The point lattices can be described by 7 axial systems (coordinate systems) z c β a x 19/1 -10 α γ b y Axial system Axes Angles Triclinic a≠b≠c α≠β≠γ≠ 90 o Monoclinic a≠b≠c α=γ=90 o ≠ β Orthorombic a≠b≠c α= β=γ=90 o Tetragonal a=b≠c α= β=γ=90 o Cubic a=b=c α= β=γ=90 o Hexagonal a 1=a 2=a 3≠c α= β=90 o γ=120 o Rhombohedral a=b=c α= β=γ ≠ 90 o MENA 3100

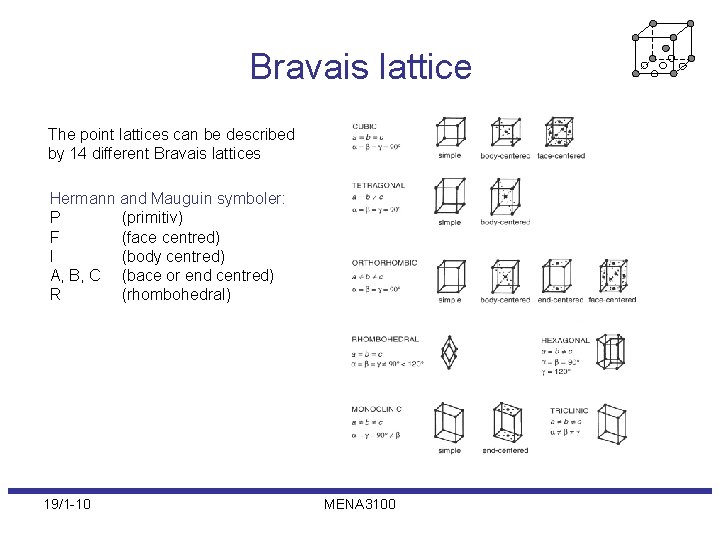
Bravais lattice The point lattices can be described by 14 different Bravais lattices Hermann and Mauguin symboler: P (primitiv) F (face centred) I (body centred) A, B, C (bace or end centred) R (rhombohedral) 19/1 -10 MENA 3100

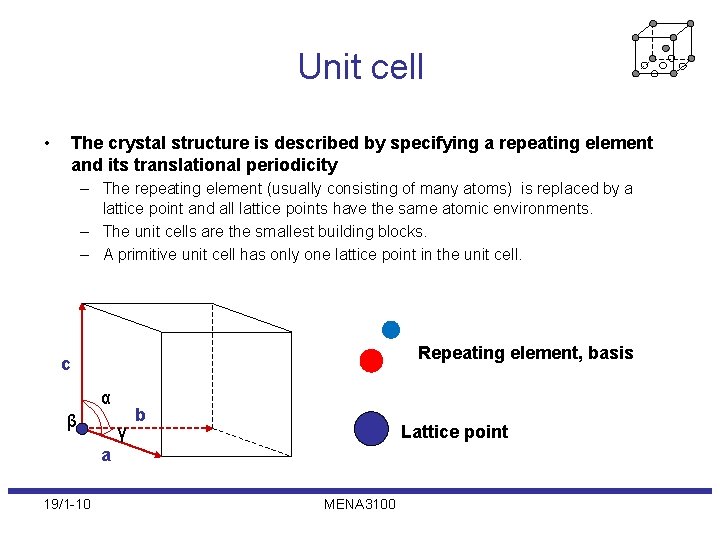
Unit cell • The crystal structure is described by specifying a repeating element and its translational periodicity – The repeating element (usually consisting of many atoms) is replaced by a lattice point and all lattice points have the same atomic environments. – The unit cells are the smallest building blocks. – A primitive unit cell has only one lattice point in the unit cell. Repeating element, basis c α β γ b Lattice point a 19/1 -10 MENA 3100

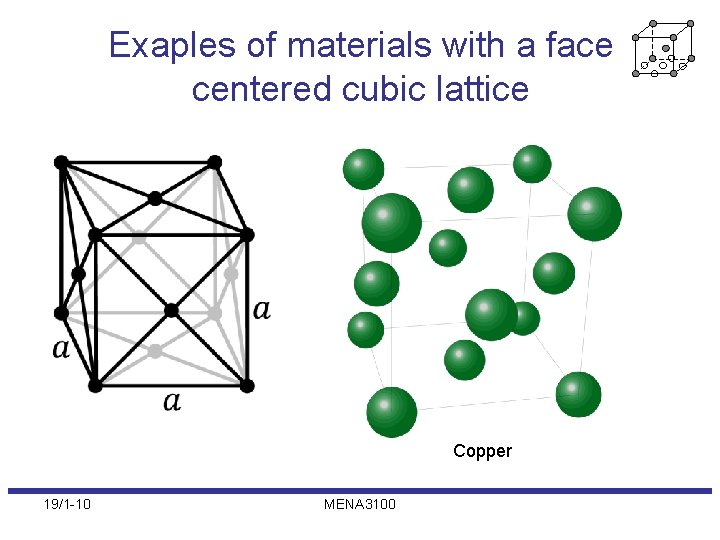
Exaples of materials with a face centered cubic lattice Copper 19/1 -10 MENA 3100

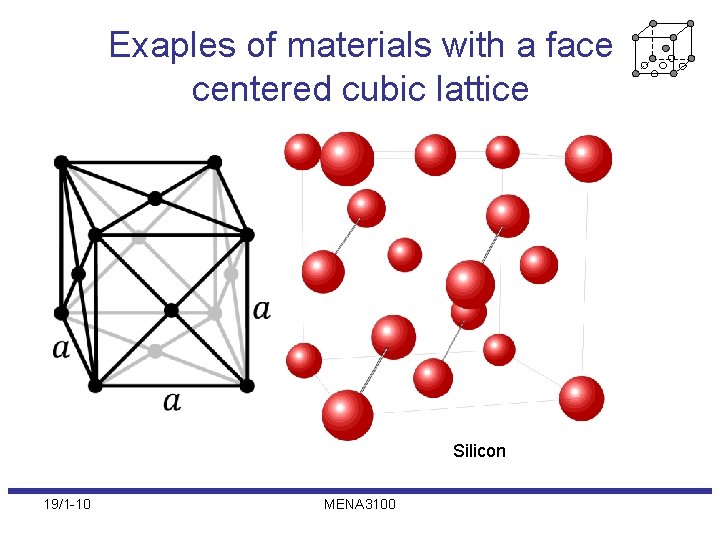
Exaples of materials with a face centered cubic lattice Silicon 19/1 -10 MENA 3100

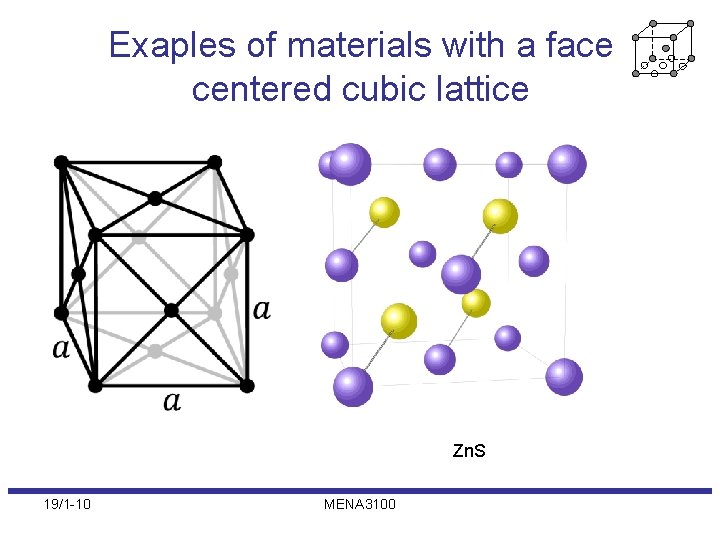
Exaples of materials with a face centered cubic lattice Zn. S 19/1 -10 MENA 3100

What about other symmetry elements? • We have discussed translational symmetry, but there also other important symmetry operations: – – – Mirror planes Rotation axes Inversion Screw axes Glide planes • The combination of these symmetry operations with the Bravais lattices give the 230 space groups 19/1 -10 MENA 3100

Space groups • Crystals can be classified according to 230 space groups. • Details about crystal description can be found in International Tables for Crystallography. – Criteria for filling Bravais point lattice with atoms. • • Structural data for known crystalline phases are available in books like “Pearson’s handbook of crystallographic data…. ” but also electronically in databases like “Find it”. • Pearson symbol like c. F 4 indicate the axial system (cubic), centering of the lattice (face) and number of atoms in the unit cell of a phase (like Cu). A space group can be referred to by a number or the space group symbol (ex. Fm-3 m is nr. 225) 19/1 -10 MENA 3100

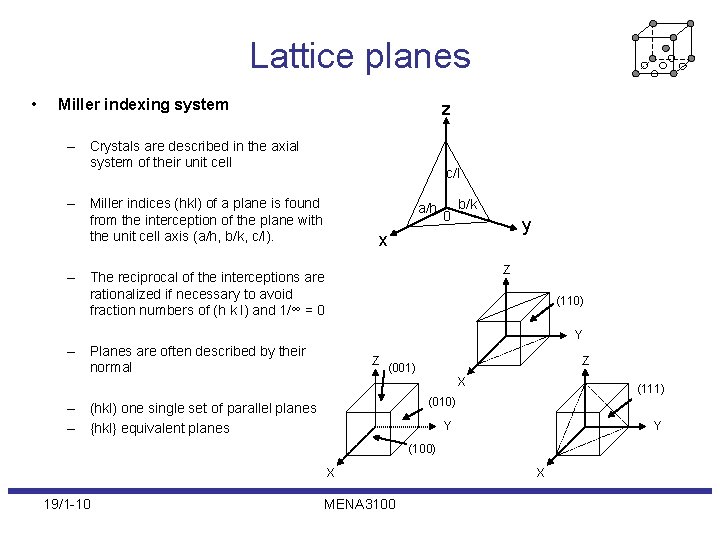
Lattice planes • Miller indexing system z – Crystals are described in the axial system of their unit cell c/l – Miller indices (hkl) of a plane is found from the interception of the plane with the unit cell axis (a/h, b/k, c/l). a/h 0 b/k y x Z – The reciprocal of the interceptions are rationalized if necessary to avoid fraction numbers of (h k l) and 1/∞ = 0 (110) Y – Planes are often described by their normal Z Z (001) X (111) (010) – (hkl) one single set of parallel planes – {hkl} equivalent planes Y Y (100) X 19/1 -10 MENA 3100 X

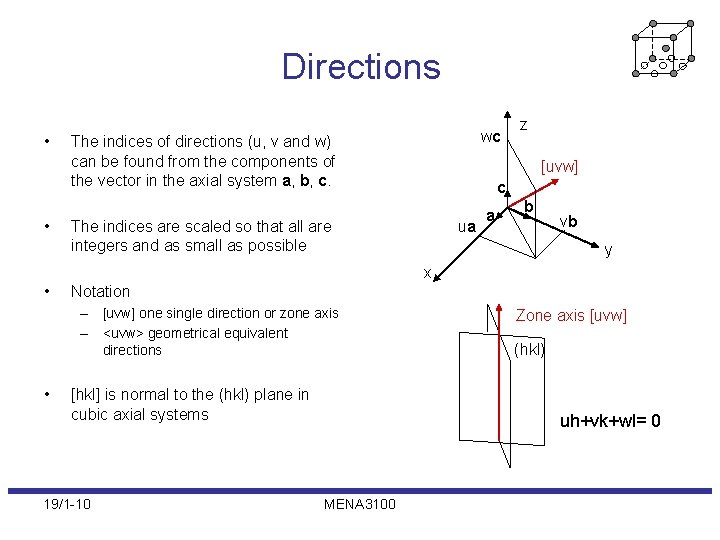
Directions • • wc The indices of directions (u, v and w) can be found from the components of the vector in the axial system a, b, c. z [uvw] c The indices are scaled so that all are integers and as small as possible ua a b vb y x • Notation – [uvw] one single direction or zone axis – <uvw> geometrical equivalent directions • [hkl] is normal to the (hkl) plane in cubic axial systems 19/1 -10 Zone axis [uvw] (hkl) uh+vk+wl= 0 MENA 3100

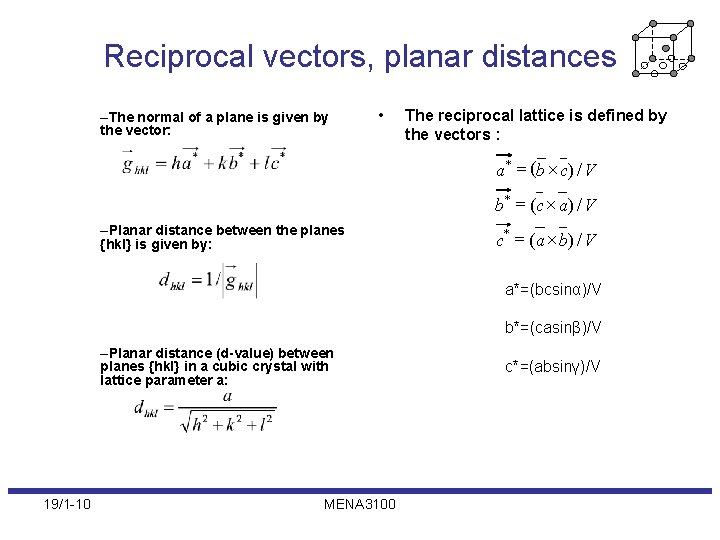
Reciprocal vectors, planar distances –The normal of a plane is given by the vector: • The reciprocal lattice is defined by the vectors : a * = (b ´ c ) / V b * = (c ´ a ) / V –Planar distance between the planes {hkl} is given by: c * = (a ´ b) / V a*=(bcsinα)/V b*=(casinβ)/V –Planar distance (d-value) between planes {hkl} in a cubic crystal with lattice parameter a: 19/1 -10 MENA 3100 c*=(absinγ)/V