Measurements and Calculations Units of measurement metric conversions




































- Slides: 74

Measurements and Calculations Units of measurement, metric conversions, arithmetic with units, conversion factors (dimensional analysis), accuracy and precision, significant figures in measurements, calculations with sig figs, scientific notation, qualitative vs. quantitative data, direct vs. indirect relations, inferences vs. observations

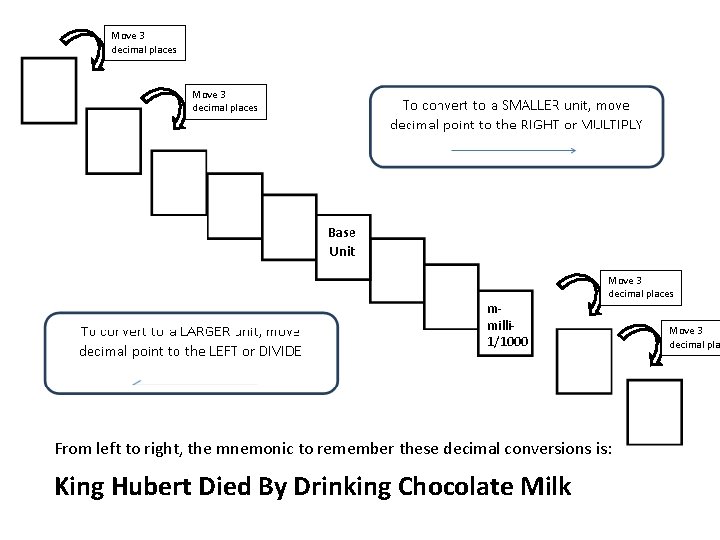
Move 3 decimal places mmilli 1/1000 Move 3 decimal places From left to right, the mnemonic to remember these decimal conversions is: King Hubert Died By Drinking Chocolate Milk Move 3 decimal pla

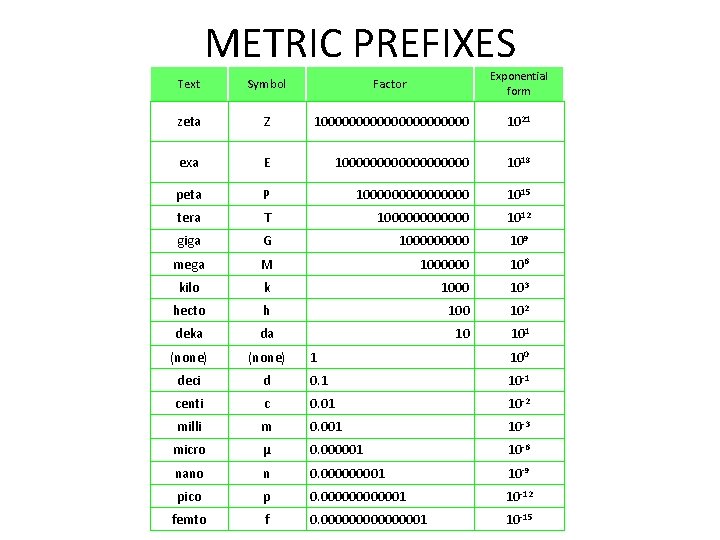
METRIC PREFIXES Text Symbol Factor Exponential form zeta Z 100000000000 1021 exa E 1000000000 1018 peta P 100000000 1015 tera T 1000000 1012 giga G 100000 109 mega M 1000000 106 kilo k 1000 103 hecto h 100 102 deka da 10 101 (none) deci 1 100 d 0. 1 10 -1 centi c 0. 01 10 -2 milli m 0. 001 10 -3 micro μ 0. 000001 10 -6 nano n 0. 00001 10 -9 pico p 0. 0000001 10 -12 femto f 0. 00000001 10 -15

Metric Prefixes A mnemonic for the first letters of the prefixes for positive exponents is Decadent Hector Killed Meg's Gigantic Terrier A mnemonic for the first letters of the prefixes for negative exponents is Darn Clever Mnemonic Makes No Prefix Forgettable

Metric Conversions To convert a number, follow these steps: 1) On the ladder, locate the prefix of the original unit (for example, milli- for mm or m. L or mg) 2) Next, locate the prefix of the desired unit (for example, kilofor km or k. L or kg) 3) Count the number of steps from the original to the desired unit (milli- to kilo- is six steps) 4) Did you go left or right to get from the original to the desired unit? a. If left, move the decimal point to the LEFT that number of places b. If right, move the decimal point to the RIGHT that number of places

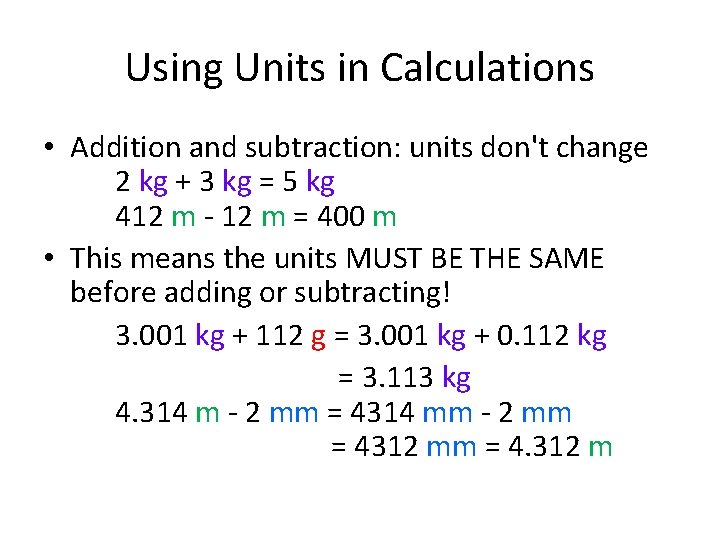
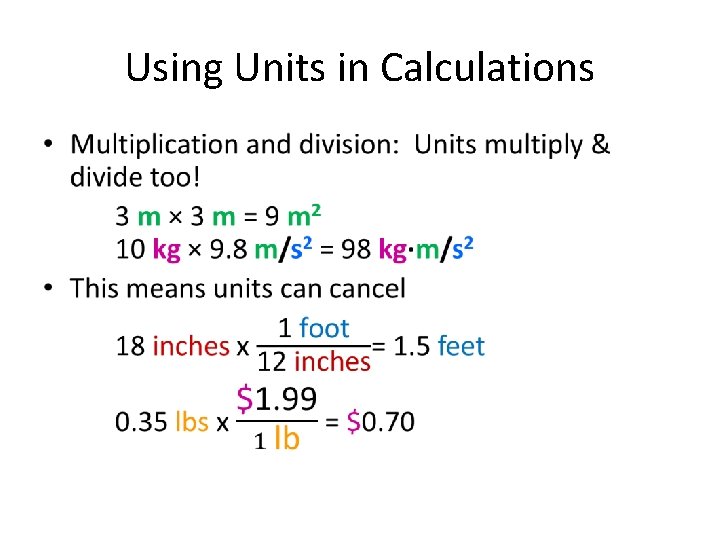
Using Units in Calculations • Addition and subtraction: units don't change 2 kg + 3 kg = 5 kg 412 m - 12 m = 400 m • This means the units MUST BE THE SAME before adding or subtracting! 3. 001 kg + 112 g = 3. 001 kg + 0. 112 kg = 3. 113 kg 4. 314 m - 2 mm = 4314 mm - 2 mm = 4312 mm = 4. 312 m

Using Units in Calculations •

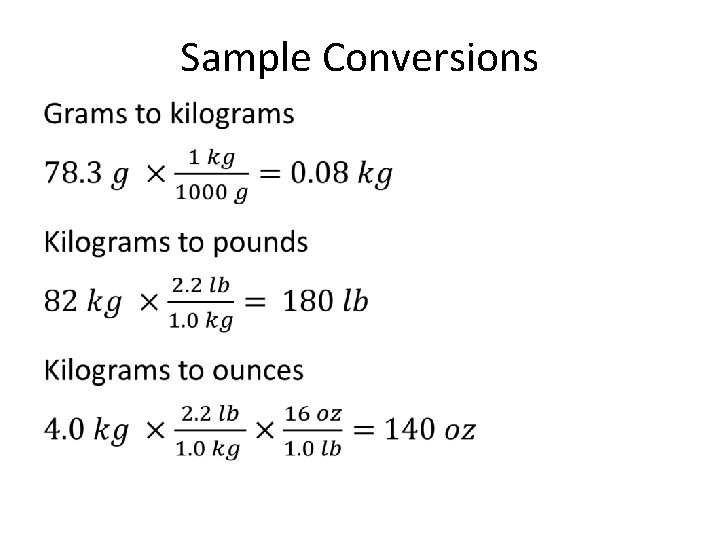
Conversions Among Units You are used to many basic conversions: • Inches to centimeters • Pounds to kilograms • Degrees Fahrenheit to degrees Celsius But what if you didn’t have your handy phone to look up a conversion program? ? We use CONVERSION FACTORS (and so do conversion programs) to make conversions among units, like 2. 54 cm = 1 inch 2. 2 lbs = 1 kg

Sample Conversions •

Conversion Factors • 5 step plan for converting units 1. 2. 3. 4. identify the unknown, including units choose a starting point list the connecting conversion factors multiply the starting measurement by conversion factors 5. Check the result: does the answer make sense?

More examples 1) 5. 0 gal/day = ? m. L/min = 13. 14 m. L/min 2) 100 sq. in = ? sq. meters = 0. 0645 sq. meters

More Complicated Conversions • Did you ever wonder how many miles are in a light year? (A light year is the distance light travels in a year. Scientists measure distances to stars in light years. ) • Distance is speed multiplied by time. Light travels at 3. 0 x 108 meters/sec. A year is 365. 25 days long. A mile is 1. 6 km. • We use these and other conversion factors to answer this question.

More Complicated Conversions •

Homework (due Friday 8/21) (20 points) Convert the following. Show all work for credit. 1) 1, 000 min = ? years 2) 92, 000 mg = ? pounds 3) 75 miles/hour = ? meters/sec 4) 8. 3 square yards = ? square cm (i. e. , cm 2)

Uncertainty in Measurements Accuracy, Precision, and Significant Figures

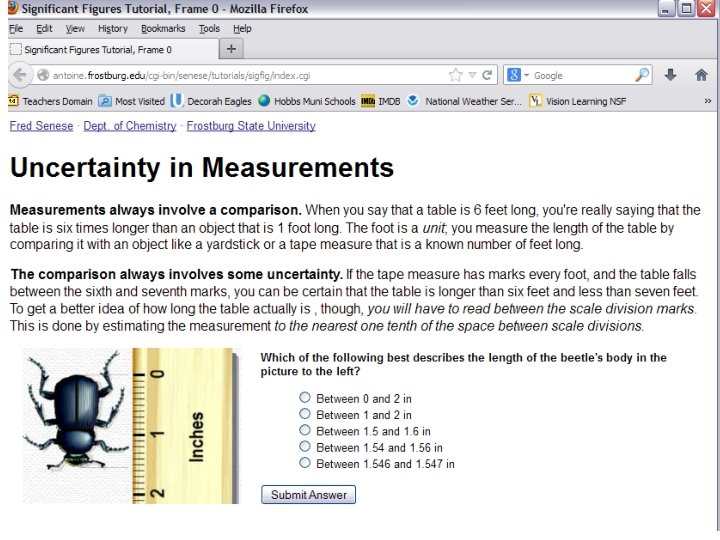
How do we handle uncertainty in measurements?


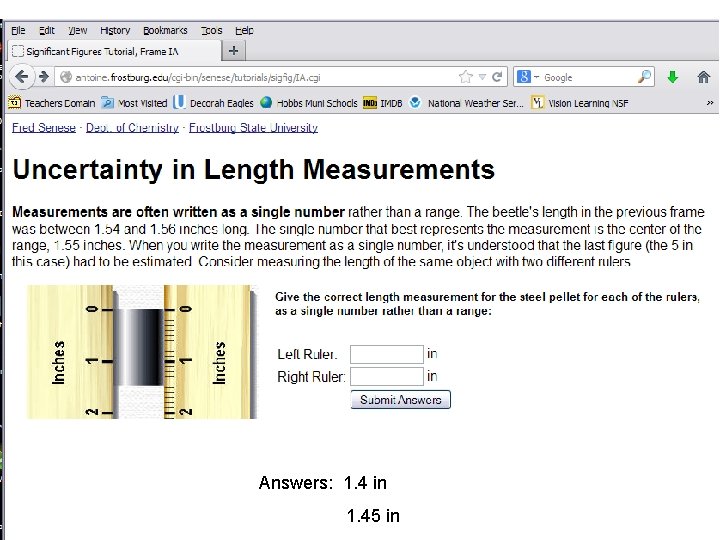
Answers: 1. 4 in 1. 45 in

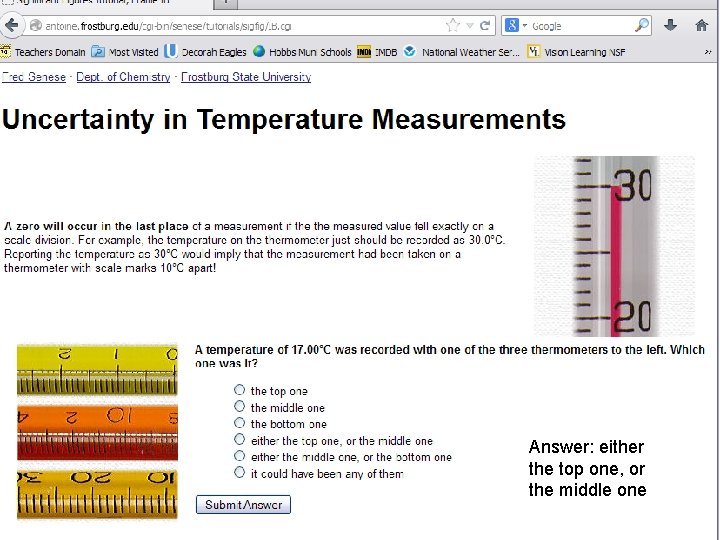
Answer: either the top one, or the middle one

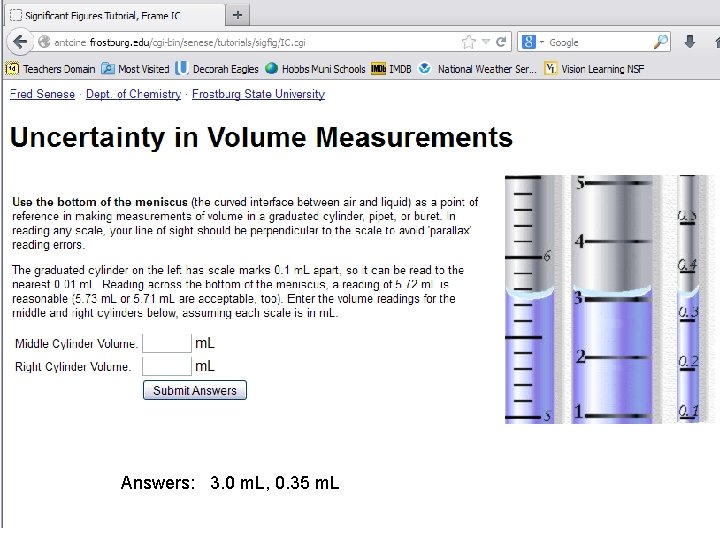
Answers: 3. 0 m. L, 0. 35 m. L


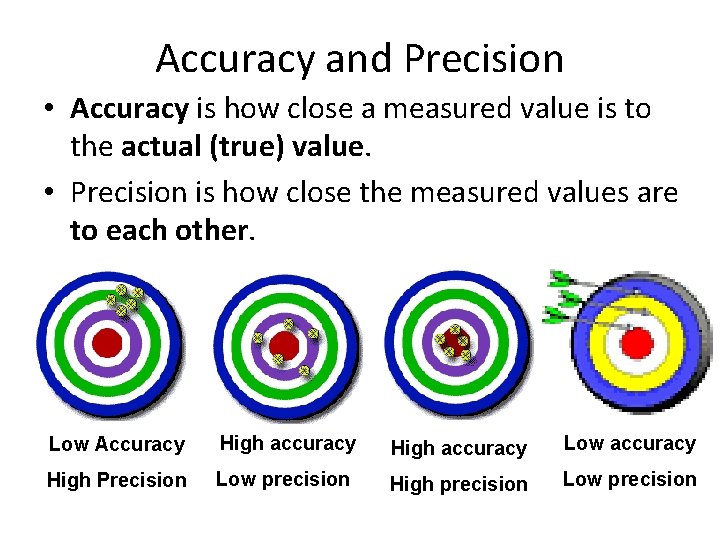
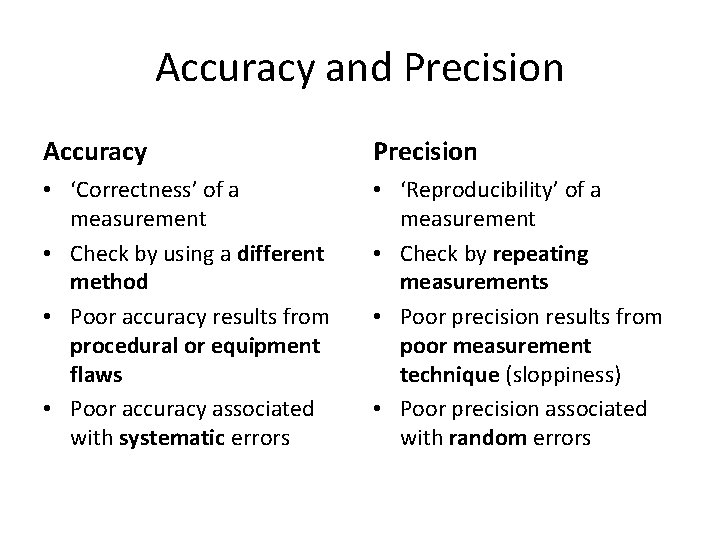
Accuracy and Precision • Accuracy is how close a measured value is to the actual (true) value. • Precision is how close the measured values are to each other. Low Accuracy High accuracy Low accuracy High Precision Low precision High precision Low precision

Accuracy and Precision Accuracy Precision • ‘Correctness’ of a measurement • Check by using a different method • Poor accuracy results from procedural or equipment flaws • Poor accuracy associated with systematic errors • ‘Reproducibility’ of a measurement • Check by repeating measurements • Poor precision results from poor measurement technique (sloppiness) • Poor precision associated with random errors

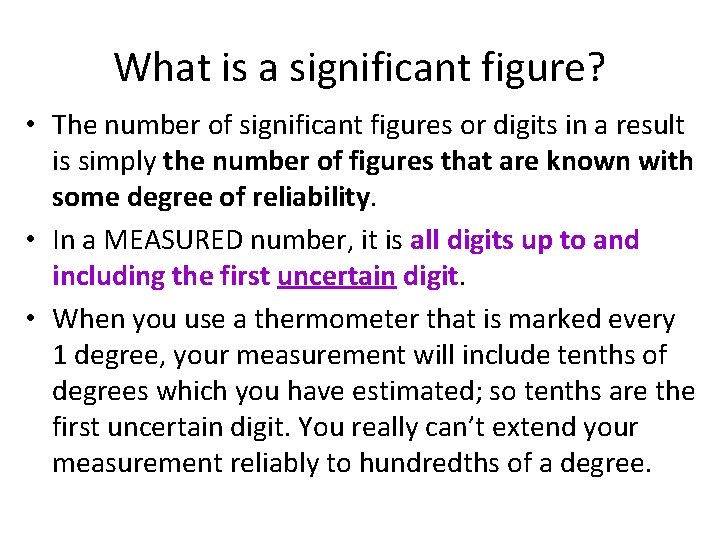
What is a significant figure? • The number of significant figures or digits in a result is simply the number of figures that are known with some degree of reliability. • In a MEASURED number, it is all digits up to and including the first uncertain digit. • When you use a thermometer that is marked every 1 degree, your measurement will include tenths of degrees which you have estimated; so tenths are the first uncertain digit. You really can’t extend your measurement reliably to hundredths of a degree.

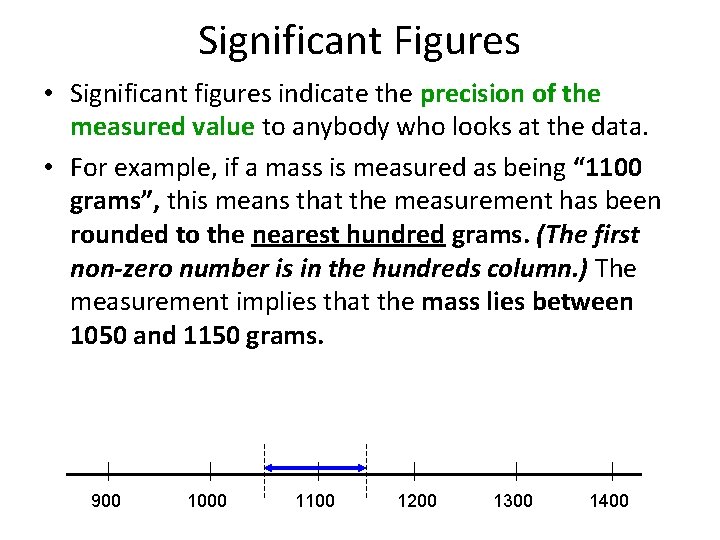
Significant Figures • Significant figures indicate the precision of the measured value to anybody who looks at the data. • For example, if a mass is measured as being “ 1100 grams”, this means that the measurement has been rounded to the nearest hundred grams. (The first non-zero number is in the hundreds column. ) The measurement implies that the mass lies between 1050 and 1150 grams. 900 1000 1100 1200 1300 1400

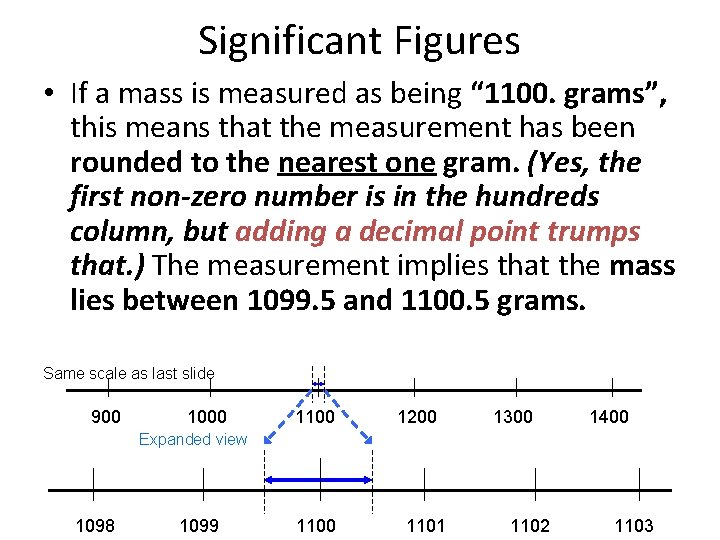
Significant Figures • If a mass is measured as being “ 1100. grams”, this means that the measurement has been rounded to the nearest one gram. (Yes, the first non-zero number is in the hundreds column, but adding a decimal point trumps that. ) The measurement implies that the mass lies between 1099. 5 and 1100. 5 grams. Same scale as last slide 900 1000 1100 1200 1300 1400 Expanded view 1098 1099 1100 1101 1102 1103

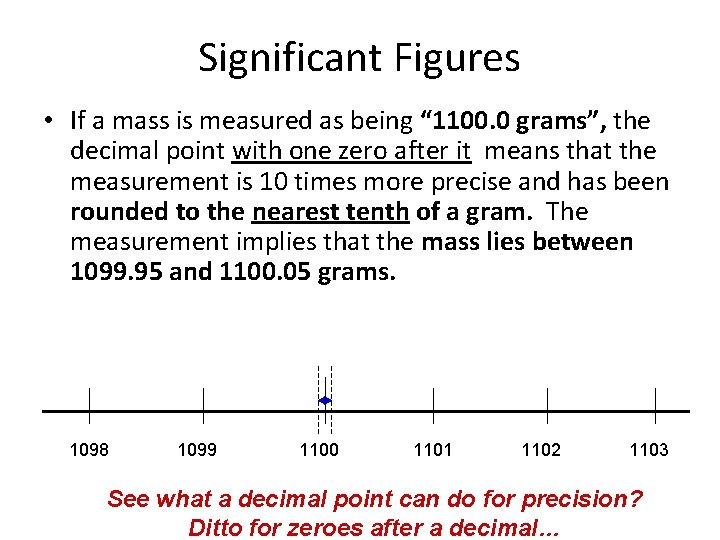
Significant Figures • If a mass is measured as being “ 1100. 0 grams”, the decimal point with one zero after it means that the measurement is 10 times more precise and has been rounded to the nearest tenth of a gram. The measurement implies that the mass lies between 1099. 95 and 1100. 05 grams. 1098 1099 1100 1101 1102 1103 See what a decimal point can do for precision? Ditto for zeroes after a decimal…

Sig Fig Rules 1. All non-zero digits are considered significant. For example, 91 has two significant figures (9 and 1), 123. 45 has five significant figures (1, 2, 3, 4 and 5). 2. Zeros between non-zero digits are significant. 101. 12 has five significant figures: 1, 0, 1, 1 and 2.

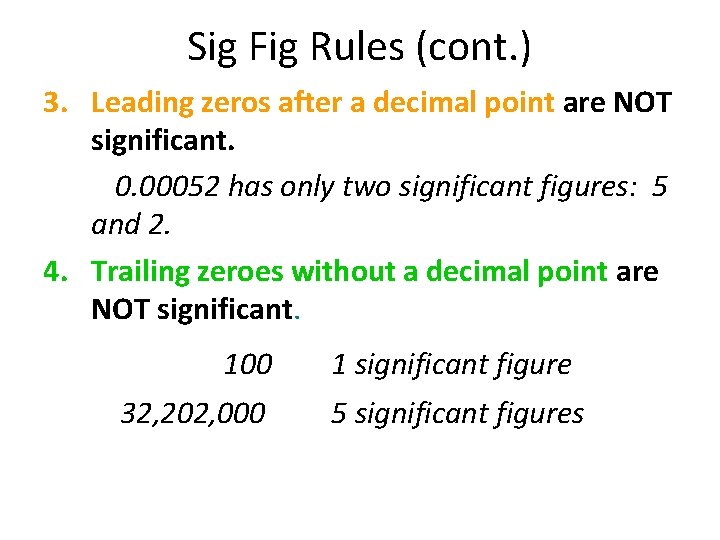
Sig Fig Rules (cont. ) 3. Leading zeros after a decimal point are NOT significant. 0. 00052 has only two significant figures: 5 and 2. 4. Trailing zeroes without a decimal point are NOT significant. 100 32, 202, 000 1 significant figure 5 significant figures

Sig Fig Rules (cont. ) 5. All digits to the LEFT of a decimal point are significant. 100 1 significant figure 100. 3 significant figures 6. Trailing zeros to the right of a decimal point are significant. 12. 2300 6 significant figures 0. 000122300 6 significant figures 102. 03 5 significant figures

Trailing Zeroes • The significance of trailing zeros in a number not containing a decimal point can be ambiguous (unclear). • For example, it may not always be clear if a number like 1300 is precise to the nearest unit (and just happens coincidentally to be an exact multiple of a hundred) or if it is only shown to the nearest hundred due to rounding or uncertainty. Do you mean 1300 (2 sig figs), or 1300. (4 sig figs), or even 1300. 0 (5 sig figs)? ? ?

How to Be Unambiguous about Trailing Zeroes • A decimal point may be placed after the number For example "100. " indicates specifically that three significant figures are meant. • Using scientific notation can eliminate the trailing zero confusion. 1300 to four significant figures is written as 1. 300× 103, while 1300 to two significant figures is written as 1. 3× 103.

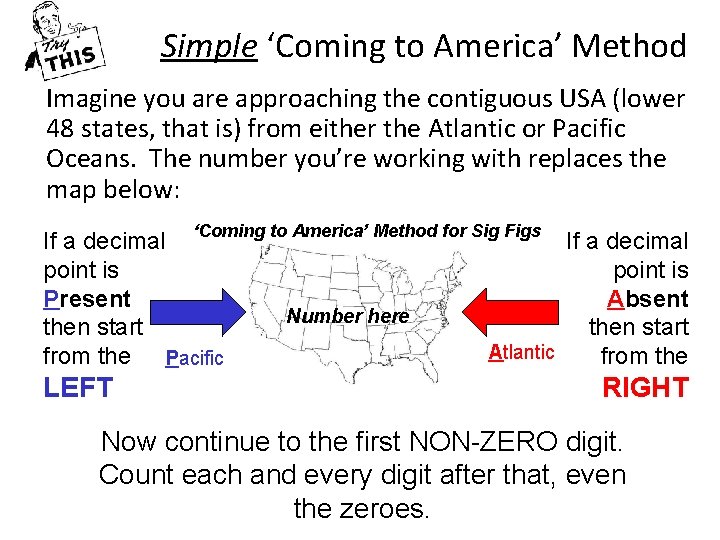
Simple ‘Coming to America’ Method Imagine you are approaching the contiguous USA (lower 48 states, that is) from either the Atlantic or Pacific Oceans. The number you’re working with replaces the map below: If a decimal ‘Coming to America’ Method for Sig Figs If a decimal point is Present Absent Number here then start Atlantic from the Pacific from the LEFT RIGHT Now continue to the first NON-ZERO digit. Count each and every digit after that, even the zeroes.

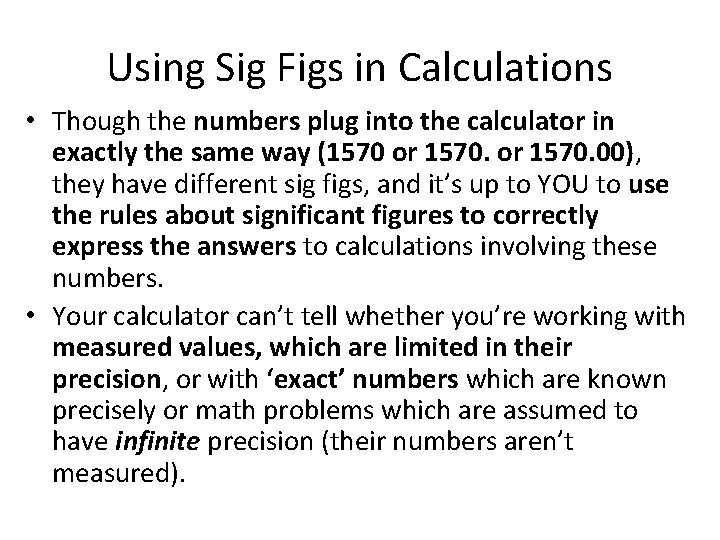
Using Sig Figs in Calculations • Okay, now you know how to recognize how many significant figures a number has. • How do you apply this knowledge to calculations so that your answer has the correct number of significant figures? • You’re probably planning on using a calculator, so next I’ll discuss some issues that arise when using this labor-saving device.

Using Sig Figs in Calculations • Though the numbers plug into the calculator in exactly the same way (1570 or 1570. 00), they have different sig figs, and it’s up to YOU to use the rules about significant figures to correctly express the answers to calculations involving these numbers. • Your calculator can’t tell whether you’re working with measured values, which are limited in their precision, or with ‘exact’ numbers which are known precisely or math problems which are assumed to have infinite precision (their numbers aren’t measured).

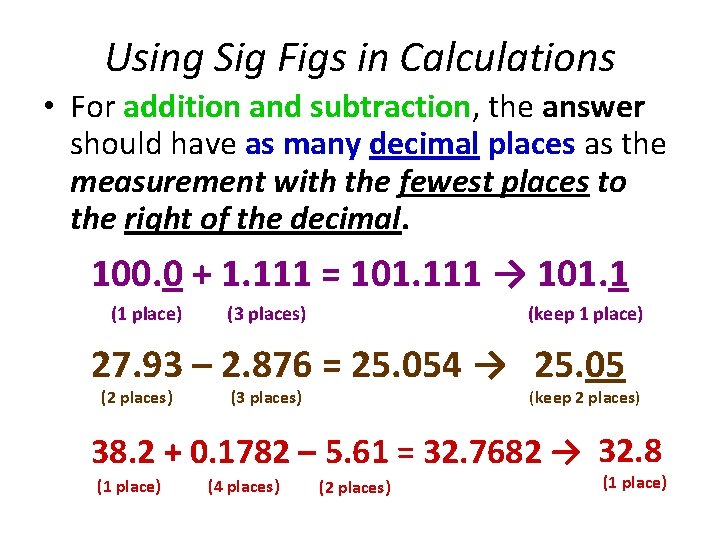
Using Sig Figs in Calculations • For addition and subtraction, the answer should have as many decimal places as the measurement with the fewest places to the right of the decimal. 100. 0 + 1. 111 = 101. 111 → 101. 1 (1 place) (3 places) (keep 1 place) 27. 93 – 2. 876 = 25. 054 → 25. 05 (2 places) (3 places) (keep 2 places) 38. 2 + 0. 1782 – 5. 61 = 32. 7682 → 32. 8 (1 place) (4 places) (2 places) (1 place)

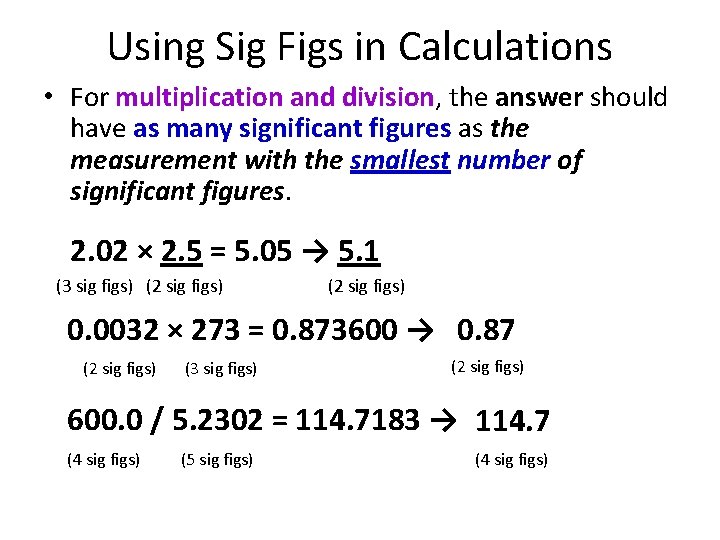
Using Sig Figs in Calculations • For multiplication and division, the answer should have as many significant figures as the measurement with the smallest number of significant figures. 2. 02 × 2. 5 = 5. 05 → 5. 1 (3 sig figs) (2 sig figs) 0. 0032 × 273 = 0. 873600 → 0. 87 (2 sig figs) (3 sig figs) (2 sig figs) 600. 0 / 5. 2302 = 114. 7183 → 114. 7 (4 sig figs) (5 sig figs) (4 sig figs)

Using Sig Figs in Calculations But what if the numerator had been 600 instead of 600. 0? 600 / 5. 2302 = 114. 7183 → (1 sig fig) (5 sig figs) (1 sig fig) Our answer has only one sig fig. This means we start at the left (because our answer has a decimal point) and keep only ONE digit (hundreds place)—and use zeroes as place holders for the tens place and the ones place. 114. 7183 becomes 100 (double check—does your answer have the correct number of sig figs? )

So when do you round a number? • When performing a calculation, do not follow these guidelines for intermediate results; keep as many digits as is practical until the end of calculation to avoid rounding errors. Consider sig figs at the END of the calculation.

Special Note re Calculators • Remember, your calculator ‘assumes’ all numbers have infinite precision IF YOU LET IT. You must be sure you do not have it set to show too few significant figures by having the mode set to ‘float’ or ‘fix’ too few digits to the right of the decimal. If you are doing calculations that include 4 digits to the right of the decimal, you must set it to ‘float’ or ‘fix’ at least 5 (one more than the # of digits). 76. 003 x 1. 0005 = 76. 04100→ 76. 041 so your calculator should be set for ‘float’ 5 in this case (5 places to the right of the decimal). • Usually you’re better off setting the ‘float’ to be ‘auto’ or a large number, as you can figure out the sig figs yourself. Just don’t forget this step!

Objective The student will be able to: express numbers in scientific and decimal notation. Designed by Skip Tyler, Varina High School

How wide is our universe? 210, 000, 000, 000 miles (22 zeros) This number is written in decimal notation. When numbers get this large, it is easier to write them in scientific notation.

Scientific Notation A number is expressed in scientific notation when it is in the form a x 10 n where a is a real number greater than or equal to 1 and less than 10 (called the coefficient) and n is an integer with 10 as the base

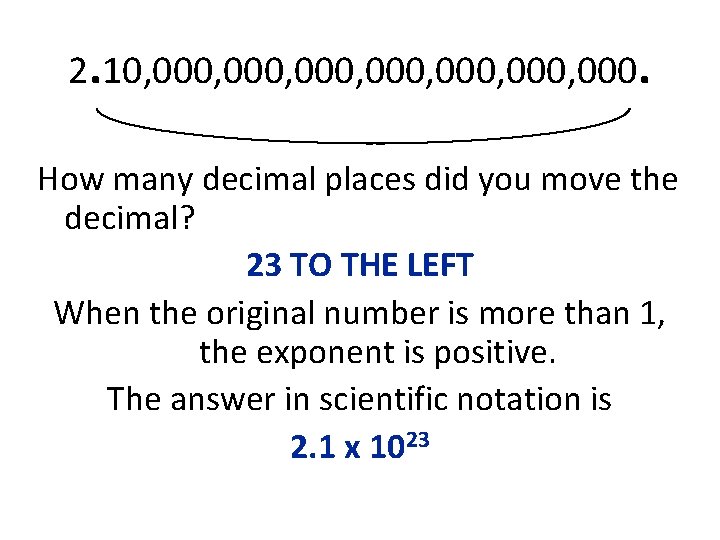
Write the width of the universe in scientific notation. 210, 000, 000, 000 miles Where is the decimal point now? After the last zero. Where would you put the decimal to make this number be between 1 and 10? Between the 2 and the 1

2. 10, 000, 000, 000. How many decimal places did you move the decimal? 23 TO THE LEFT When the original number is more than 1, the exponent is positive. The answer in scientific notation is 2. 1 x 1023

1) Express 0. 0000000902 in scientific notation. Where would the decimal go to make the number be between 1 and 10? 9. 02 The decimal was moved how many places? 8 TO THE RIGHT When the original number is less than 1, the exponent is negative. 9. 02 x 10 -8

Write 28750. 9 in scientific notation. 1. 2. 3. 4. 2. 87509 x 10 -5 2. 87509 x 10 -4 2. 87509 x 105

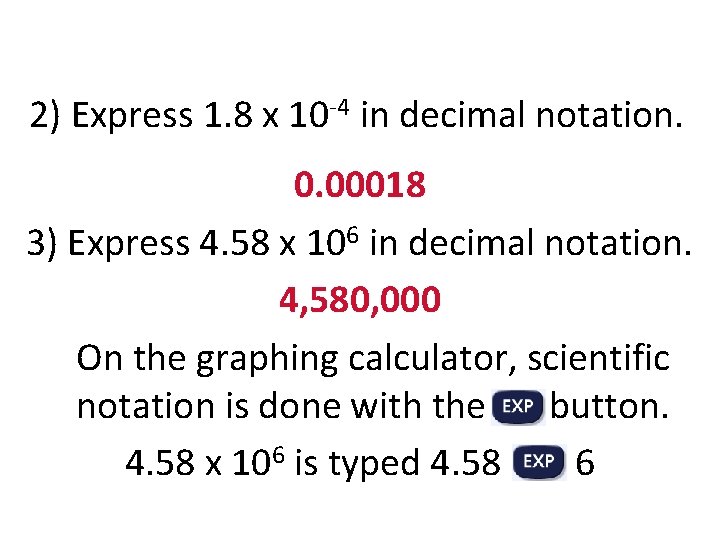
2) Express 1. 8 x 10 -4 in decimal notation. 0. 00018 3) Express 4. 58 x 106 in decimal notation. 4, 580, 000 On the graphing calculator, scientific notation is done with the button. 4. 58 x 106 is typed 4. 58 6

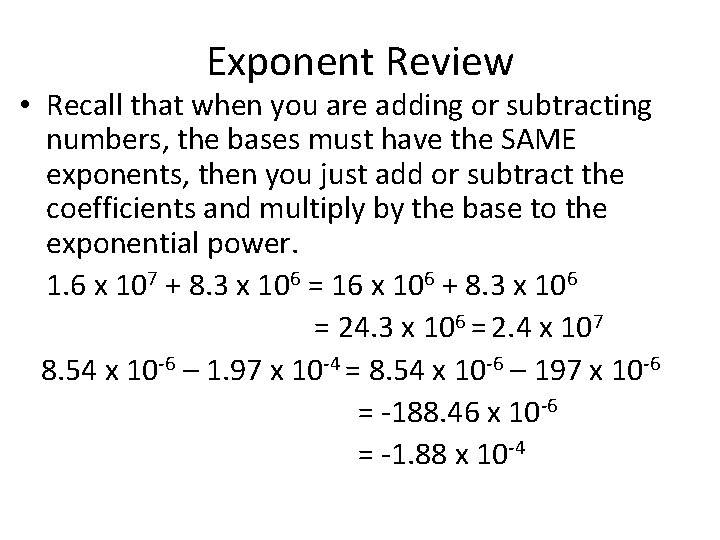
Exponent Review • Recall that when you are adding or subtracting numbers, the bases must have the SAME exponents, then you just add or subtract the coefficients and multiply by the base to the exponential power. 1. 6 x 107 + 8. 3 x 106 = 16 x 106 + 8. 3 x 106 = 24. 3 x 106 = 2. 4 x 107 8. 54 x 10 -6 – 1. 97 x 10 -4 = 8. 54 x 10 -6 – 197 x 10 -6 = -188. 46 x 10 -6 = -1. 88 x 10 -4

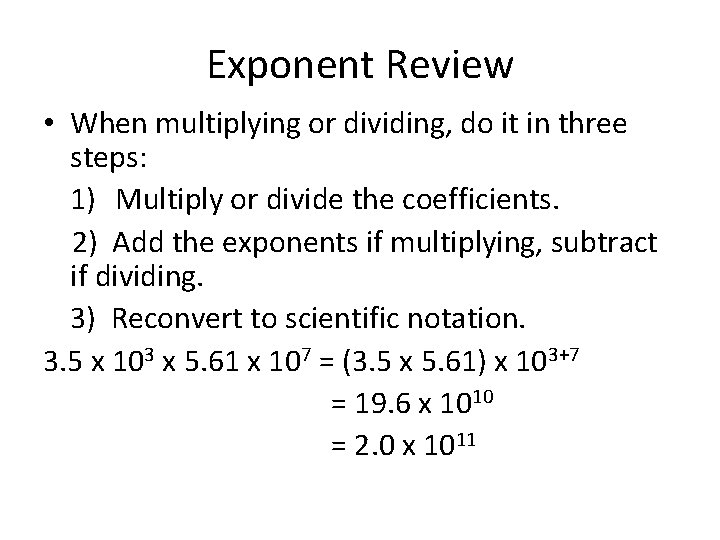
Exponent Review • When multiplying or dividing, do it in three steps: 1) Multiply or divide the coefficients. 2) Add the exponents if multiplying, subtract if dividing. 3) Reconvert to scientific notation. 3. 5 x 103 x 5. 61 x 107 = (3. 5 x 5. 61) x 103+7 = 19. 6 x 1010 = 2. 0 x 1011

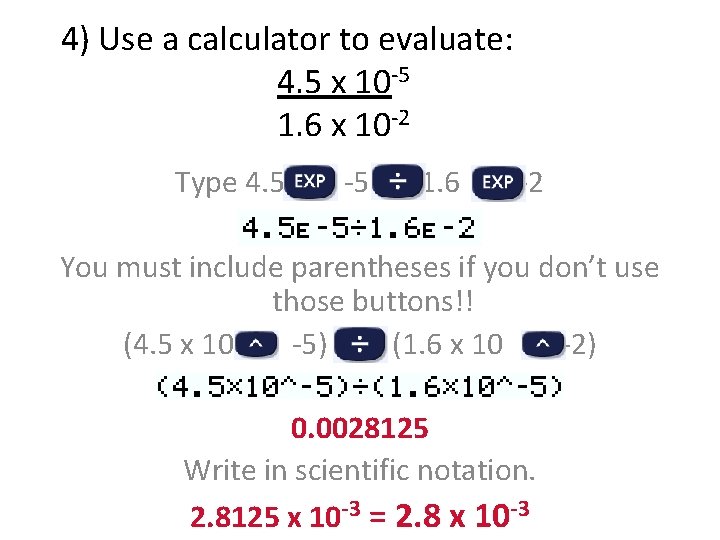
4) Use a calculator to evaluate: 4. 5 x 10 -5 1. 6 x 10 -2 Type 4. 5 -5 1. 6 -2 You must include parentheses if you don’t use those buttons!! (4. 5 x 10 -5) (1. 6 x 10 -2) 0. 0028125 Write in scientific notation. 2. 8125 x 10 -3 = 2. 8 x 10 -3

5) Use a calculator to evaluate: 7. 2 x 10 -9 1. 2 x 102 On the calculator, the answer is: 6. E -11 The answer in scientific notation is 6. 0 x 10 -11 The answer in decimal notation is 0. 0000060

6) Use a calculator to evaluate . (0. 0042)(330, 000) On the calculator, the answer is 1386. The answer in decimal notation is 1386 The answer in scientific notation is 1. 4 x 103

7) Use a calculator to evaluate (3, 600, 000)(23). On the calculator, the answer is: 8. 28 E +10 The answer in scientific notation is 8. 3 x 10 10 The answer in decimal notation is 83, 000, 000

Write (2. 8 x 103)(5. 1 x 10 -7) in scientific notation. 1. 2. 3. 4. 14 x 10 -4 1. 4 x 10 -3 14 x 1010 1. 4 x 1011

Write in PROPER scientific notation. (Notice the number is not between 1 and 10) 8) 234. 6 x 109 2. 346 x 1011 4 9) 0. 0642 x 10 on calculator: 642 6. 42 x 10 2

Write 531. 42 x 105 in scientific notation. 1. 2. 3. 4. 5. 6. 7. . 53142 x 102 5. 3142 x 103 53. 142 x 104 531. 42 x 105 53. 142 x 106 5. 3142 x 107. 53142 x 108

Measurements and Calculations We have reviewed metric units of measurement, performed density calculations, contrasted observations with inferences, discussed accuracy and precision, learned how to apply the rules for significant figures to measurements, and how to express standard numbers in scientific notation and vice versa. But we’re not quite done with the subject of measurement and calculations…

Weight vs. Mass • Are mass and weight the same thing? We tend to use the words interchangeably but they are NOT. • Mass is a measure of the quantity of matter. • Any two masses exert a mutual attractive force on each other which we call gravity. The amount of that force is weight and depends on the masses and how far apart they are. Unlike weight, mass does NOT depend on gravity.

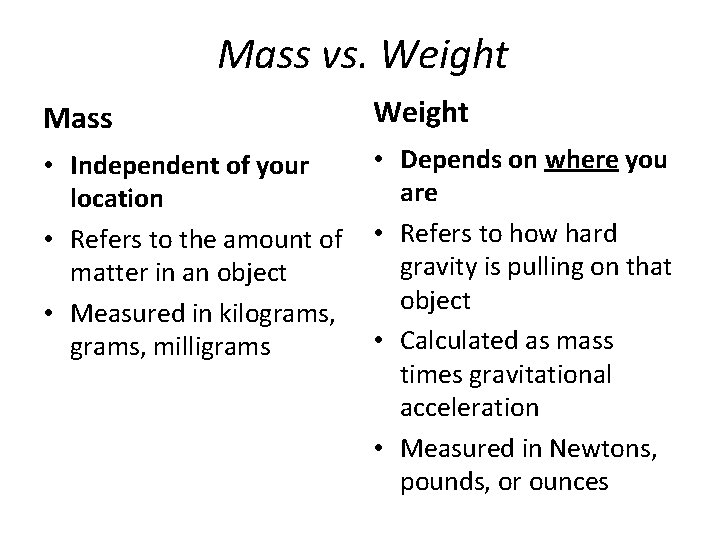
Mass vs. Weight Mass Weight • Depends on where you • Independent of your are location • Refers to the amount of • Refers to how hard gravity is pulling on that matter in an object • Measured in kilograms, • Calculated as mass grams, milligrams times gravitational acceleration • Measured in Newtons, pounds, or ounces

Gravitational Force = Weight • A one kilogram mass on the Earth's surface results in 2. 2 pounds of force between the mass and the Earth, so we say the mass weighs 2. 2 pounds. • That same one kilogram mass on the Moon, because of the Moon's lower mass, results in only about 1/3 pound of mutual force. • On the ‘surface’ of the sun, however, that same 1 kilogram mass weighs 61. 5 pounds!

Inertial property of mass • Can you push a locomotive over? • No, of course not, it has too much inertia, a property of mass that resists changes in motion. • If that locomotive was floating in space, far from any celestial bodies, could you push it and make it move? • No, because its mass is still the same, and it resists being moved. Weightlessness has nothing to do with it.

Qualitative vs. Quantitative Data • Ms. Hancock has a male Irish Wolfhound mixed breed dog who is 12 years old, weighs 85 pounds, is mostly brown with a white chest and feet and some black markings, and hates riding in the car. • Which data are quantitative? • Which data are qualitative? Go to hyperlink →

DIRECT Proportions • Two quantities are directly proportional to each other if dividing one by the other gives a constant value. • Density is an example of this kind of relationship, as mass divided by volume is a constant for a particular substance.


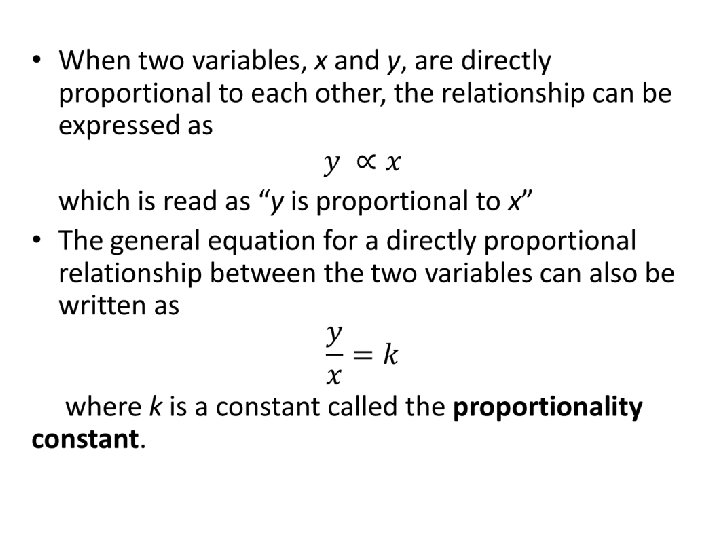
Linear Relationships • ALL DIRECTLY PROPORTIONAL RELATIONSHIPS PRODUCE LINEAR GRAPHS THAT PASS THROUGH THE ORIGIN.

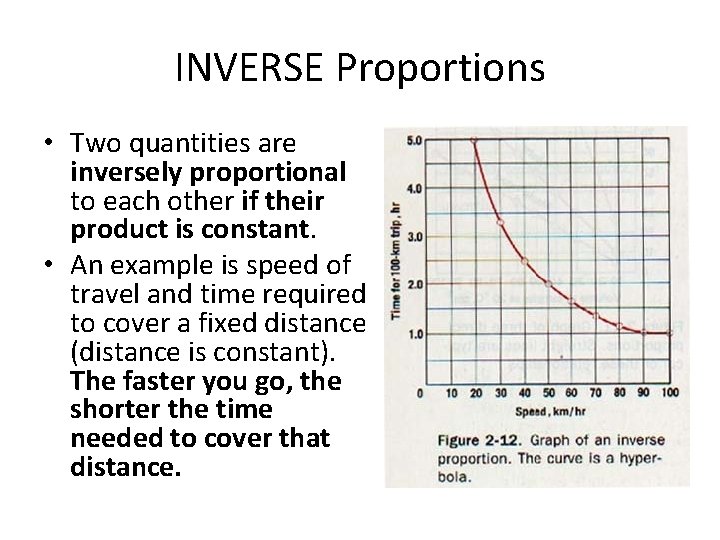
INVERSE Proportions • Two quantities are inversely proportional to each other if their product is constant. • An example is speed of travel and time required to cover a fixed distance (distance is constant). The faster you go, the shorter the time needed to cover that distance.


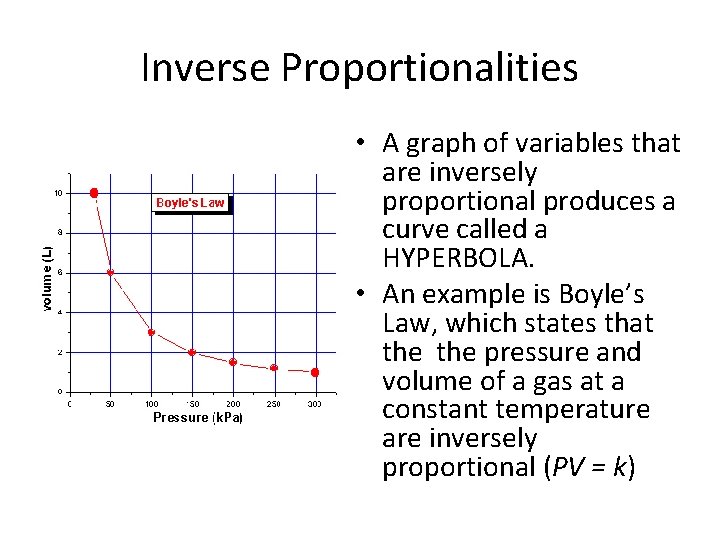
Inverse Proportionalities • A graph of variables that are inversely proportional produces a curve called a HYPERBOLA. • An example is Boyle’s Law, which states that the pressure and volume of a gas at a constant temperature are inversely proportional (PV = k)

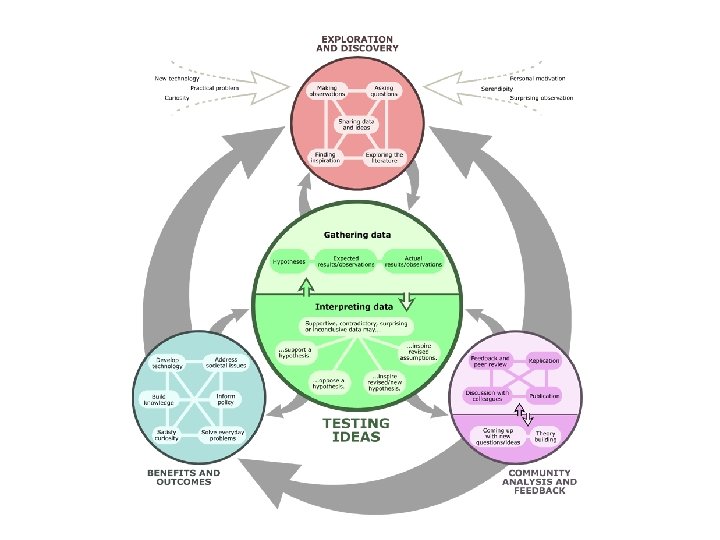
Scientific Method • While sometimes science progresses by accidental discoveries, most of the time it is by carefully planned investigations using the Scientific Method. • “A logical approach to solving problem by observing and collecting data, formulating hypotheses, testing hypotheses, and formulating theories that are supported by data. ”

Hypotheses as Testable Statements • We discussed a hypothesis as an ‘educated guess’ or a testable statement which serves as a basis for making predictions and for carrying out further experiments. • Testing a hypothesis requires experimentation to produce or otherwise collecting evidence (data) to support or refute it. During testing, the conditions that remain constant are called controls, and any condition that changes is called a variable.

Models and Theories • When the data support a hypothesis, scientists usually try to explain the phenomena they are studying by constructing a model. • A model in science is more than a physical object or mental picture; it is an explanation of how phenomena occur and how data or events are related. It can be visual, verbal or mathematical. • If a model is very successful at explaining many phenomena, it may become part of a theory, a broad generalization that explains a body of facts or phenomena.

But are experiments the only way to apply the Scientific Method? • Well, no. Not all science fits this linear model. • We already discussed that we could make inferences and formulate hypotheses from observations that could not be tested by repetition (earthquake photo, dinosaur extinction, migration into South America) • Real science is not necessarily a linear process. The scientific process can be complex and take many different paths.

 Metric mania
Metric mania 3 units of linear measurements in metric system
3 units of linear measurements in metric system Typical room height metric unit
Typical room height metric unit Metric units of measurement
Metric units of measurement Basic conversions
Basic conversions Si metric units
Si metric units Ladder method conversions
Ladder method conversions Conversions and calculations chapter 6
Conversions and calculations chapter 6 Chapter 2 measurements and calculations
Chapter 2 measurements and calculations Pharmaceutical measurements and calculations
Pharmaceutical measurements and calculations Percent error example
Percent error example Pharmacy measurements
Pharmacy measurements Scientific notation and metric conversions
Scientific notation and metric conversions Metric and household measurements
Metric and household measurements Structural steel connection calculations calculations
Structural steel connection calculations calculations Ladder of metric units
Ladder of metric units Metric ladder method
Metric ladder method Metric conversion dimensional analysis
Metric conversion dimensional analysis Write the correct abbreviation for each metric unit meter
Write the correct abbreviation for each metric unit meter Nurse metric conversion chart
Nurse metric conversion chart Metric conversion jeopardy
Metric conversion jeopardy Dimensional analysis worksheet
Dimensional analysis worksheet Metric ladder method
Metric ladder method Scientific notation king henry
Scientific notation king henry 5 physical quantities and their si units
5 physical quantities and their si units Conversion table maths lit
Conversion table maths lit Conversions of units
Conversions of units Metric system review
Metric system review English and metric units
English and metric units Metric units of mass and capacity
Metric units of mass and capacity Customary and metric units
Customary and metric units Converting customary units
Converting customary units Measurement units standards and services department
Measurement units standards and services department Metric measurement virtual lab
Metric measurement virtual lab Metric system of measurement
Metric system of measurement Googleclassroomn
Googleclassroomn What are the 7 metric units?
What are the 7 metric units? Km hec dec m dm cm mm
Km hec dec m dm cm mm Metric system notes
Metric system notes Metric units of capacity
Metric units of capacity Area in metric system
Area in metric system Metric units chemistry
Metric units chemistry The metric units of density are:
The metric units of density are: What are the advantages of the metric system?
What are the advantages of the metric system? What is the volume of this aquarium
What is the volume of this aquarium Melt thru weld symbol
Melt thru weld symbol English vs metric
English vs metric Gallon man chart
Gallon man chart Momentum units physics
Momentum units physics Customary system
Customary system Customary units of measurement
Customary units of measurement Mili centi deci deca hecto kilo
Mili centi deci deca hecto kilo Units of measurement in physics
Units of measurement in physics Xeomin reconstitution chart
Xeomin reconstitution chart Variable costing income statement
Variable costing income statement Ratios rates and conversions worksheet answers
Ratios rates and conversions worksheet answers Ratios rates and conversions
Ratios rates and conversions Thermochemistry conversions
Thermochemistry conversions Eroei
Eroei Elementary name and address conversions
Elementary name and address conversions Measurements equivalents and adjustments
Measurements equivalents and adjustments Force and torque measurements
Force and torque measurements Ee8403 measurements and instrumentation
Ee8403 measurements and instrumentation Anthropometric measurement includes vital signs
Anthropometric measurement includes vital signs Vital signs and body measurements quiz
Vital signs and body measurements quiz Angle measurements and segment lengths
Angle measurements and segment lengths Normal range for vital signs
Normal range for vital signs Vital signs and anthropometric measurements:
Vital signs and anthropometric measurements: Measurements and uncertainties ib physics
Measurements and uncertainties ib physics Measurement uncertainty
Measurement uncertainty Diamtral
Diamtral Metrology and measurements subject code
Metrology and measurements subject code Measurement abbreviations and equivalents answer key
Measurement abbreviations and equivalents answer key Using and expressing measurements
Using and expressing measurements Measurements and scientific tools lesson 2
Measurements and scientific tools lesson 2