Matter REVIEW CONCEPTS Matter is anything that has






- Slides: 16

Matter REVIEW CONCEPTS

Matter is anything that has mass and takes up space.

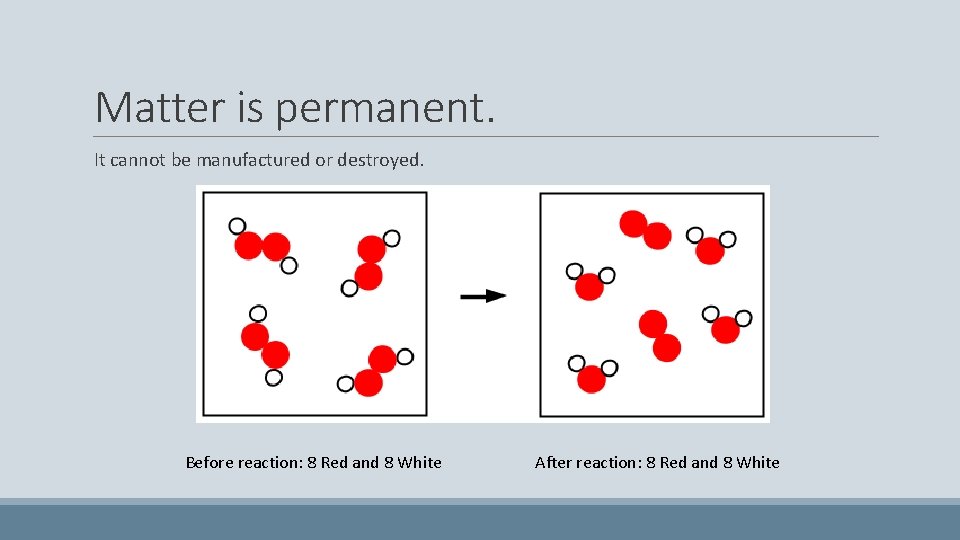
Matter is permanent. It cannot be manufactured or destroyed. Before reaction: 8 Red and 8 White After reaction: 8 Red and 8 White

Matter can be changed in two ways 1. Physical Change 2. Chemical Change

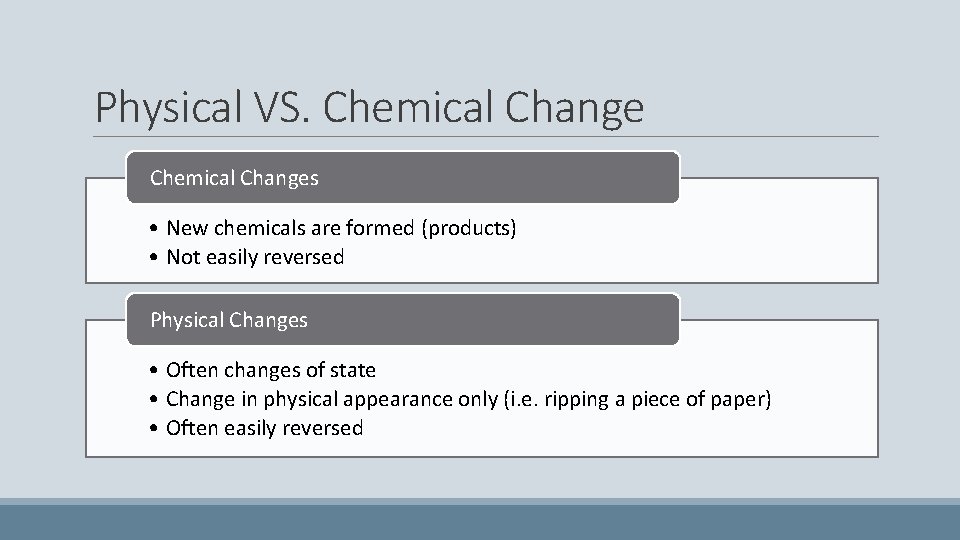
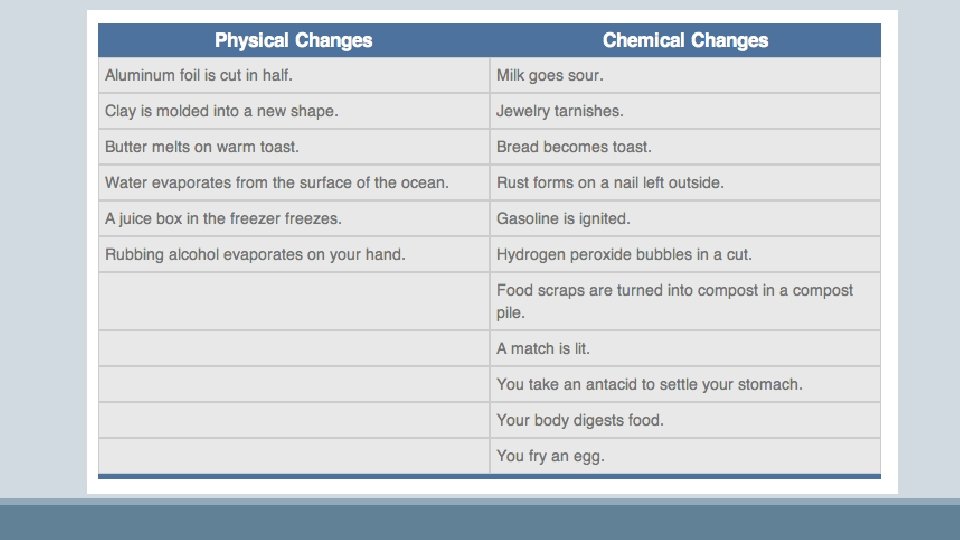
Physical VS. Chemical Changes • New chemicals are formed (products) • Not easily reversed Physical Changes • Often changes of state • Change in physical appearance only (i. e. ripping a piece of paper) • Often easily reversed


Matter exists in three states.

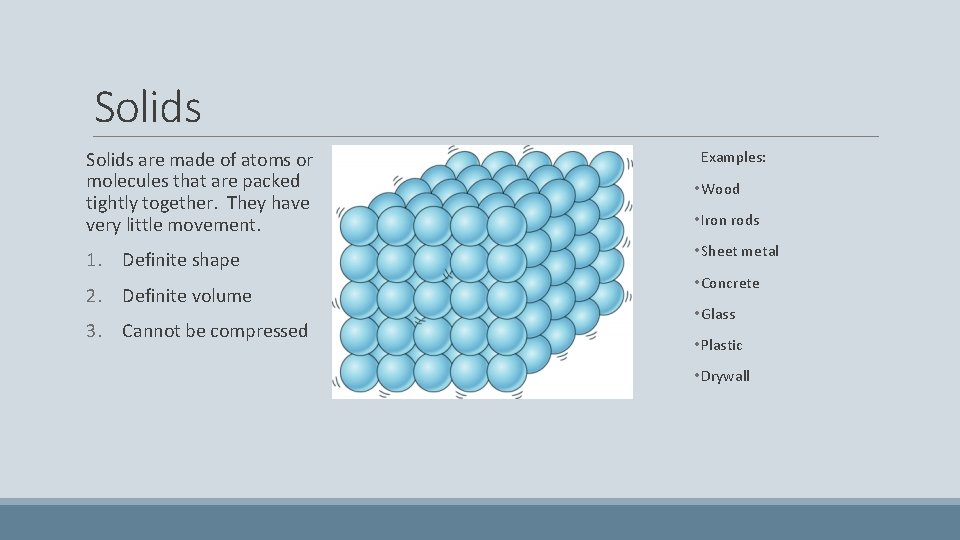
Solids are made of atoms or molecules that are packed tightly together. They have very little movement. 1. Definite shape 2. Definite volume 3. Cannot be compressed Examples: • Wood • Iron rods • Sheet metal • Concrete • Glass • Plastic • Drywall

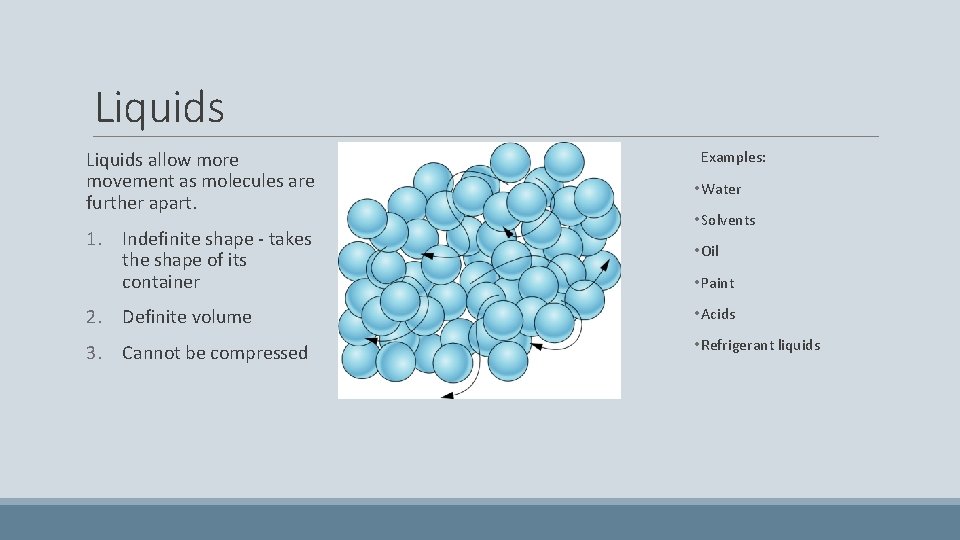
Liquids allow more movement as molecules are further apart. 1. Indefinite shape - takes the shape of its container Examples: • Water • Solvents • Oil • Paint 2. Definite volume • Acids 3. Cannot be compressed • Refrigerant liquids

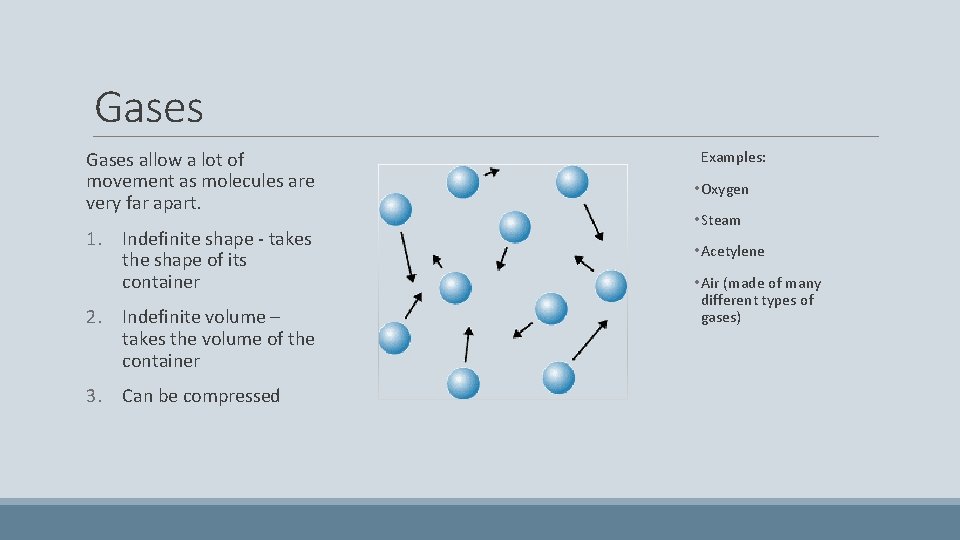
Gases allow a lot of movement as molecules are very far apart. 1. Indefinite shape - takes the shape of its container 2. Indefinite volume – takes the volume of the container 3. Can be compressed Examples: • Oxygen • Steam • Acetylene • Air (made of many different types of gases)

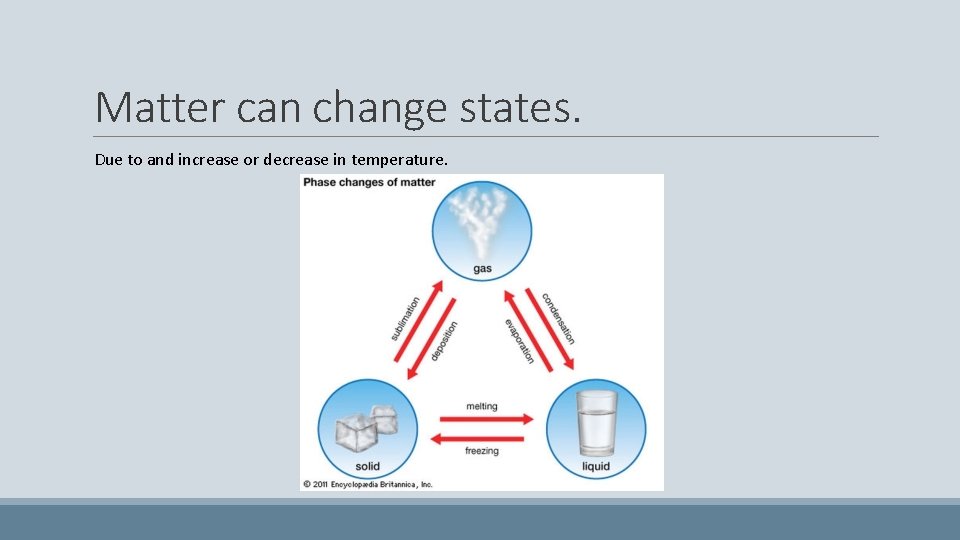
Matter can change states. Due to and increase or decrease in temperature.

Phase changes SOLID TO LIQUID Melting/Fusion LIQUID TO SOLID Freezing/Solidification

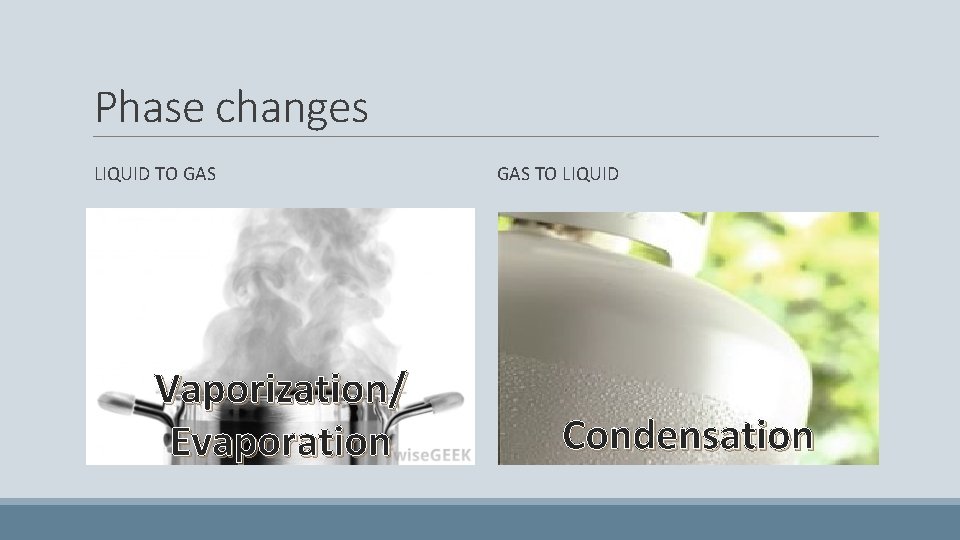
Phase changes LIQUID TO GAS Vaporization/ Evaporation GAS TO LIQUID Condensation

Phase changes SOLID TO GAS Sublimation GAS TO SOLID Crystallization/Deposition

Phase Changes Elements change phases based on temperature Every element has a different temperature where it will change state. So we know the temperatures for a substance like water? What temperature does it freeze? What temperature does it evaporate? How is this different for other substances?

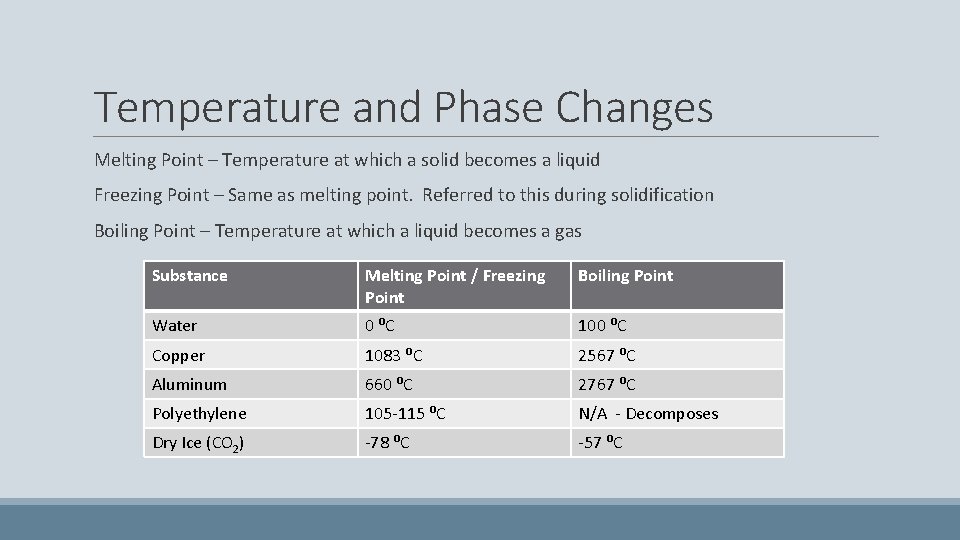
Temperature and Phase Changes Melting Point – Temperature at which a solid becomes a liquid Freezing Point – Same as melting point. Referred to this during solidification Boiling Point – Temperature at which a liquid becomes a gas Substance Melting Point / Freezing Point Boiling Point Water 0 ⁰C 100 ⁰C Copper 1083 ⁰C 2567 ⁰C Aluminum 660 ⁰C 2767 ⁰C Polyethylene 105 -115 ⁰C N/A - Decomposes Dry Ice (CO 2) -78 ⁰C -57 ⁰C
 Anything that has mass and takes up space
Anything that has mass and takes up space Anything that has mass and take up space
Anything that has mass and take up space Anything that takes up space and has mass is
Anything that takes up space and has mass is It has a mass and occupies space
It has a mass and occupies space Matter is anything that has mass and volume.
Matter is anything that has mass and volume. Anything that has matter and takes up space
Anything that has matter and takes up space Anything that takes up space and has mass is
Anything that takes up space and has mass is The matter is anything that occupies
The matter is anything that occupies Matter is anything that has
Matter is anything that has Matter is anything that has both
Matter is anything that has both Matter is anything that occupies space and has -----------
Matter is anything that occupies space and has ----------- Anything that has mass and takes up space
Anything that has mass and takes up space Ngoại tâm thu thất chùm đôi
Ngoại tâm thu thất chùm đôi Block av độ 2
Block av độ 2 Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Thơ thất ngôn tứ tuyệt đường luật
Thơ thất ngôn tứ tuyệt đường luật Chiến lược kinh doanh quốc tế của walmart
Chiến lược kinh doanh quốc tế của walmart