Matter Properties and Changes Matter Anything that has









- Slides: 15

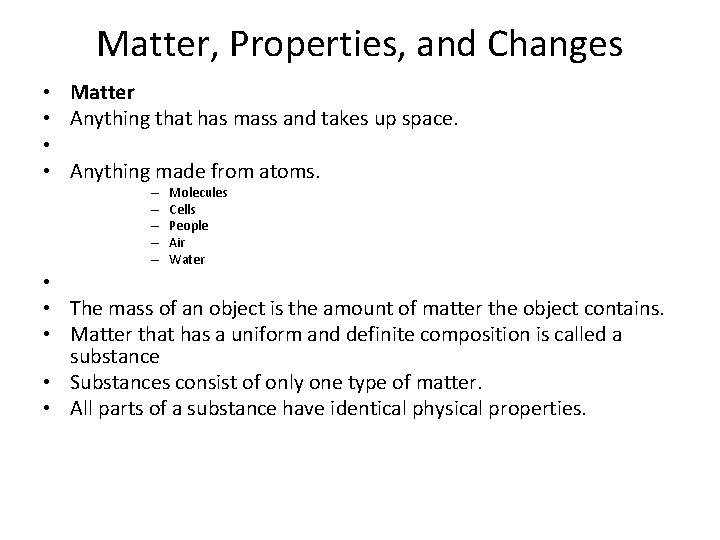
Matter, Properties, and Changes • • Matter Anything that has mass and takes up space. Anything made from atoms. – – – Molecules Cells People Air Water • • The mass of an object is the amount of matter the object contains. • Matter that has a uniform and definite composition is called a substance • Substances consist of only one type of matter. • All parts of a substance have identical physical properties.

Matter, Properties, and Changes Physical Properties • A characteristic that can be observed without changing the sample’s composition • • (1) Density • (2) Color • (3) Melting point • (4) Boiling point • (5) State of matter

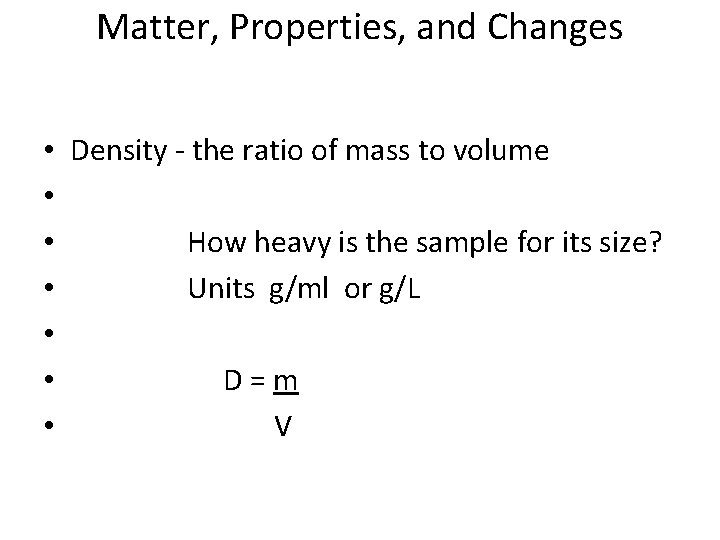
Matter, Properties, and Changes • Density - the ratio of mass to volume • • How heavy is the sample for its size? • Units g/ml or g/L • • D = m • V

Matter, Properties, and Changes • Chemical Properties: ability of a substance to combine with or change into one or more other substances – a characteristic that cannot be observed until the original sample has changed composition • (1) Ability of iron to rust • (2) Ability of silver to tarnish

Matter, Properties, and Changes • States of Matter • (1) Solid - matter with definite shape and volume • particles are packed together in a rigid arrangement; they are not free to move around • particles vibrate in place • compressibility: almost zero • • (2) Liquid - matter with definite volume but no definite shape (particles flow to take the shape of the container) particles are close to one another, but are not rigidly packed. Particles touch each other but are free to move Compressibility: almost incompressible

Matter, Properties, and Changes • (3) Gas - matter with neither definite volume or shape – Particles move freely - flow like a liquid to take the shape of the container. – Particles spaced far apart – Compressibility: very high – Expands on heating • • (I)Vapor - gaseous state of a substance that is normally

Matter, Properties, and Changes • Changes in Matter • 1. Physical change – • alter a substance without changing its composition • • A. examples: – tear paper – break glass – change in state

Matter, Properties, and Changes • • Chemical change – process of one or more substances changing into a new substance Always produces a change in properties A. examples: iron rusting food cooking B. reactants and products – reactant - starting material – product - ending material • • c. Possible indicators of a chemical change • • color change in temperature formation of a solid (precipitate) formation of a gas

Matter, Properties, and Changes • Conservation of Mass • Matter cannot be created or destroyed during a chemical reaction •

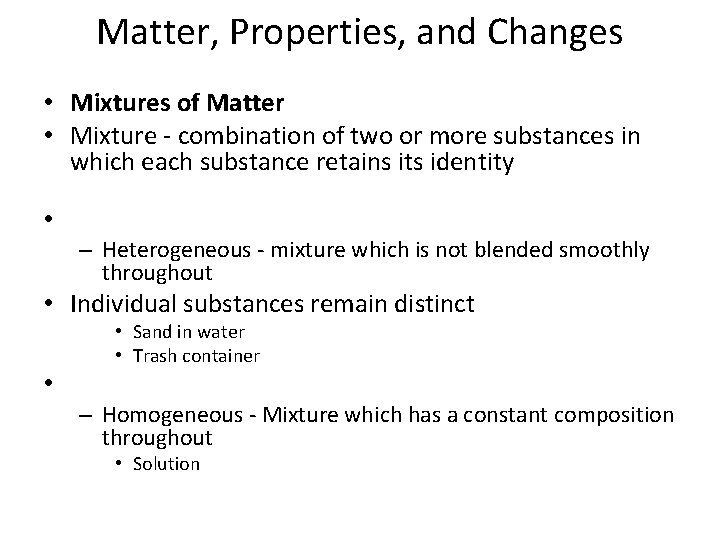
Matter, Properties, and Changes • Mixtures of Matter • Mixture - combination of two or more substances in which each substance retains its identity • – Heterogeneous - mixture which is not blended smoothly throughout • Individual substances remain distinct • • Sand in water • Trash container – Homogeneous - Mixture which has a constant composition throughout • Solution

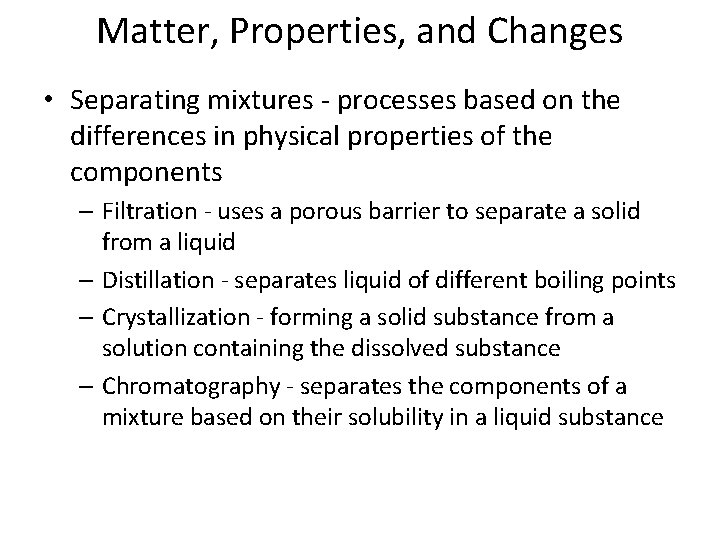
Matter, Properties, and Changes • Separating mixtures - processes based on the differences in physical properties of the components – Filtration - uses a porous barrier to separate a solid from a liquid – Distillation - separates liquid of different boiling points – Crystallization - forming a solid substance from a solution containing the dissolved substance – Chromatography - separates the components of a mixture based on their solubility in a liquid substance

Matter, Properties, and Changes • Elements and Compounds • Element - pure substance that cannot be separated into simpler substances by chemical means – Represented by one or two letter chemical symbols • • Atom - smallest part of an element containing all the properties of the element

Matter, Properties, and Changes • Compound - chemical combination of two or more elements – Chemical symbols are a way to write the formulas of compounds • Sugar - C 12 H 22 O 12 • • Molecule - smallest part of a compound having all the properties of the compound

Matter, Properties, and Changes • The Periodic Table • • Arrangement of elements according to chemical properties • • Mendeleev - devised modern periodic table

Matter, Properties, and Changes