Management of Advanced Head Neck Squamous Cell Carcinoma












- Slides: 31

Management of Advanced Head & Neck Squamous Cell Carcinoma in The Molecular Era Mohamed Abdulla (M. D. ) Department of Clinical Oncology Kasr El-Aini School of Medicine Cairo University Alexandria, 15/01/09

Epidemiology of SCCHN Squamous cell carcinoma of the head and neck (SCCHN): 98 000 new cases in Europe annually SCCHN: mortality in Europe is 43 000 annually Worldwide annual incidence of SCCHN: 485 000 new patients; 261 000 deaths SCCHN accounts for 6% of all malignancies GLOBOCAN 2002 (http: //www-dep. iar. fr)

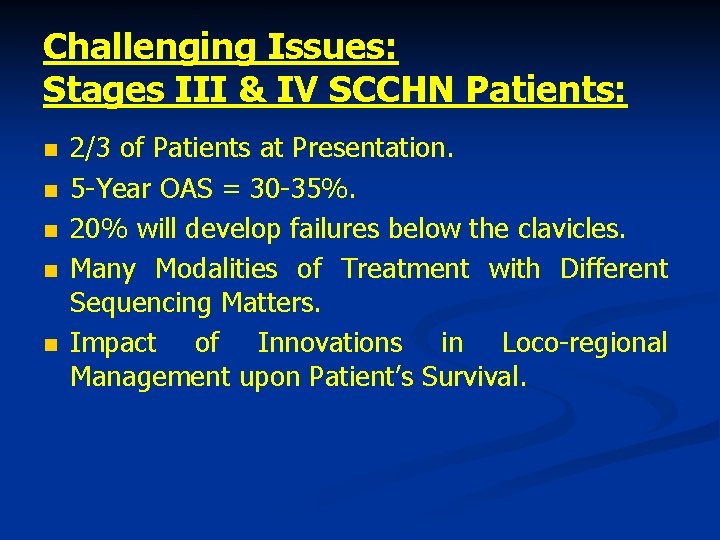
Challenging Issues: Stages III & IV SCCHN Patients: n n n 2/3 of Patients at Presentation. 5 -Year OAS = 30 -35%. 20% will develop failures below the clavicles. Many Modalities of Treatment with Different Sequencing Matters. Impact of Innovations in Loco-regional Management upon Patient’s Survival.

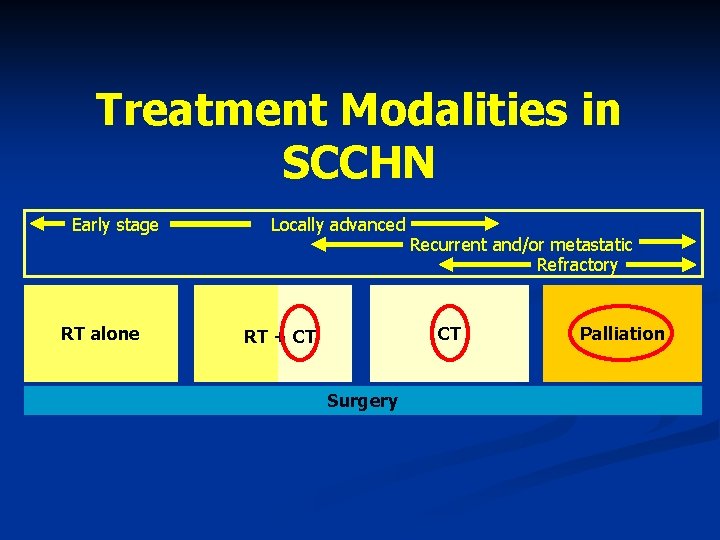
Treatment Modalities in SCCHN Early stage RT alone Locally advanced Recurrent and/or metastatic Refractory CT RT + CT Surgery Palliation

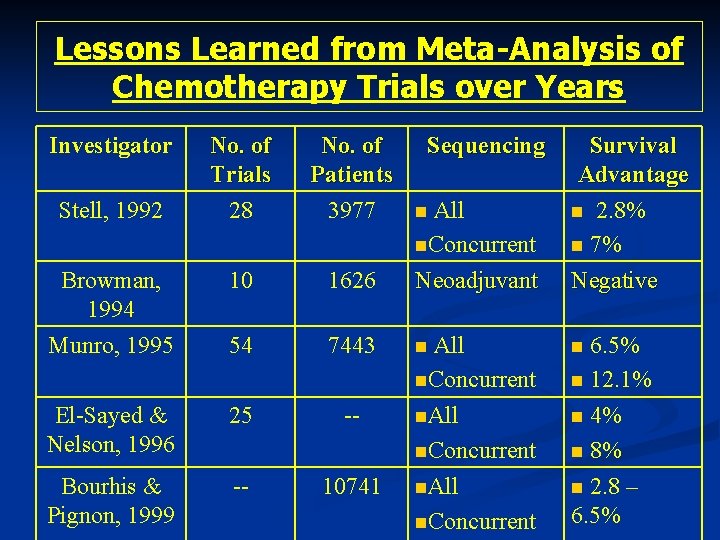
Lessons Learned from Meta-Analysis of Chemotherapy Trials over Years Investigator No. of Trials 28 No. of Patients 3977 Browman, 1994 10 1626 All n. Concurrent Neoadjuvant Munro, 1995 54 7443 n El-Sayed & Nelson, 1996 25 -- Bourhis & Pignon, 1999 -- 10741 Stell, 1992 Sequencing n All n. Concurrent n. All n. Concurrent Survival Advantage n 2. 8% n 7% Negative 6. 5% n 12. 1% n 4% n 8% n 2. 8 – 6. 5% n

Lessons Learned from Meta-Analysis of Chemotherapy Trials over Years n Ø Ø Ø Cancer Care Ontario Practice Guidelines, 2000: 18 Randomized Controlled Trials. 3192 Patients. Absolute Mortality Risk Reduction with Concurrent Cth = 11%. Absolute Mortality Risk Reduction with Monotherapy Platinum Based Cth = 12%. The Cost of Incremental Acute Toxicity.

Lessons Learned from Meta-Analysis of Chemotherapy Trials over Years Ø Ø Ø 1. 2. 3. ASCO 2004: 87 Trials. 16000 Patients. Survival Advantage: All: 5% at 5 y. Concurrent: 11% at 5 y. Platinum Monotherapy ASCO 2007

Lessons Learned from Meta-Analysis of Chemotherapy Trials over Years n n n Concurrent Chemotherapy Improves Survival by 8 -11%. Platinum Monotherapy is Preferred. Little Role in Pure Neoadjuvant or Adjuvant Fashions.

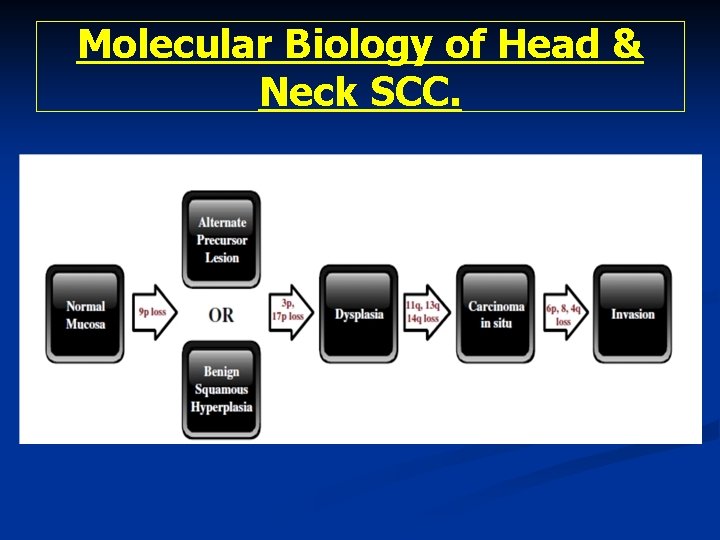
Molecular Biology of Head & Neck SCC.


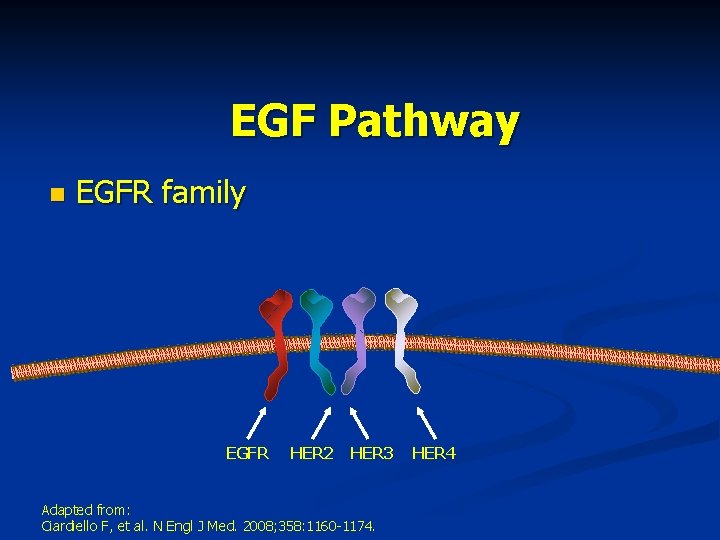
EGF Pathway n EGFR family EGFR HER 2 HER 3 Adapted from: Ciardiello F, et al. N Engl J Med. 2008; 358: 1160 -1174. HER 4

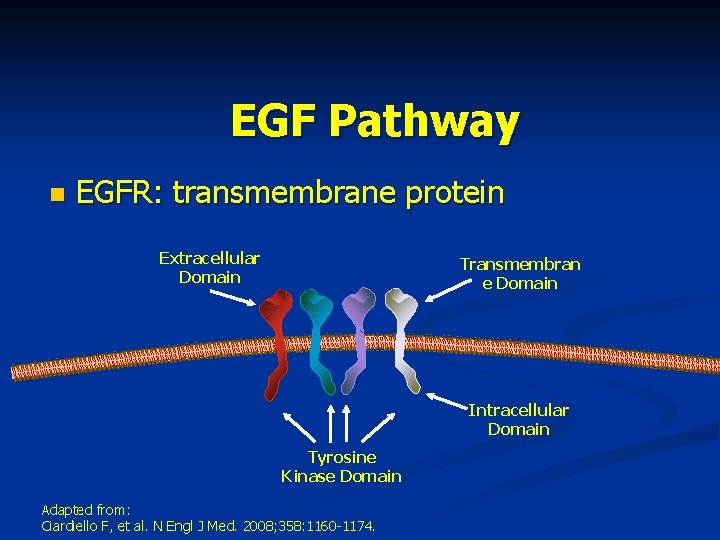
EGF Pathway n EGFR: transmembrane protein Extracellular Domain Transmembran e Domain Intracellular Domain Tyrosine Kinase Domain Adapted from: Ciardiello F, et al. N Engl J Med. 2008; 358: 1160 -1174.

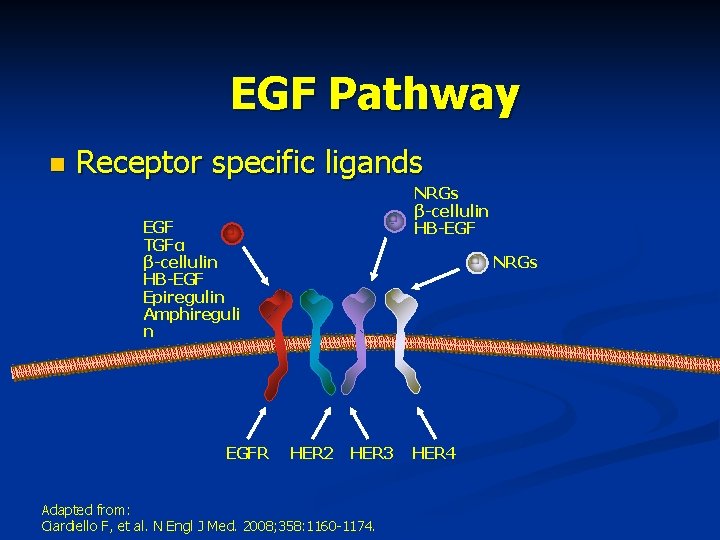
EGF Pathway n Receptor specific ligands NRGs β-cellulin HB-EGF TGFα β-cellulin HB-EGF Epiregulin Amphireguli n EGFR NRGs HER 2 HER 3 Adapted from: Ciardiello F, et al. N Engl J Med. 2008; 358: 1160 -1174. HER 4

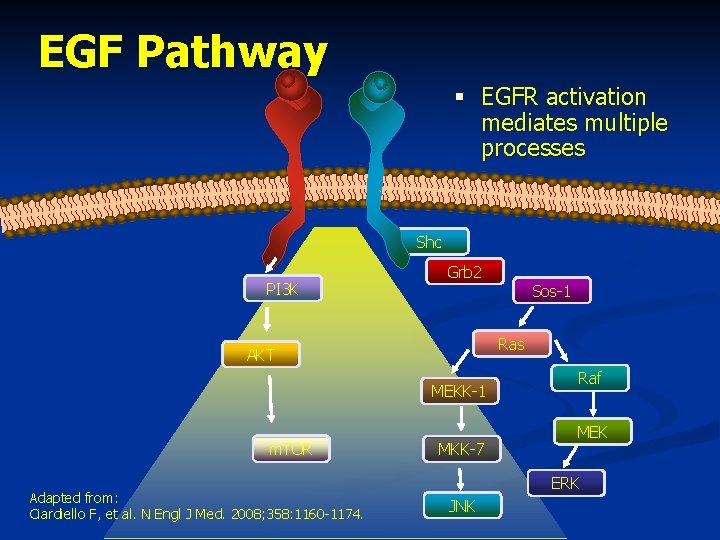
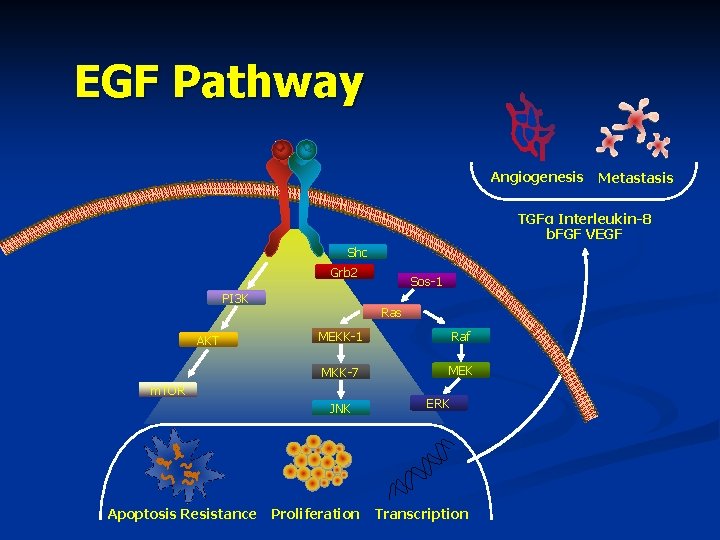
EGF Pathway § EGFR activation mediates multiple processes Shc PI 3 K Grb 2 Ras AKT MEKK-1 m. TOR Adapted from: Ciardiello F, et al. N Engl J Med. 2008; 358: 1160 -1174. Sos-1 MKK-7 Raf MEK ERK JNK

EGF Pathway Angiogenesis Metastasis TGFα Interleukin-8 b. FGF VEGF Shc Grb 2 PI 3 K AKT Ras MEKK-1 Raf MKK-7 MEK m. TOR JNK Apoptosis Resistance Sos-1 Proliferation ERK Transcription

Prognostic & Predictive Importance of EGFR Over expression: n n > 90% of all HNSCC Patients. Poor Response to ttt with Chemo-Radiotherapy Through Repopulation of Clonogenic Cells during ttt. Compromised L. C. , DFS, OAS. Associated with Cisplatin-Resistance.


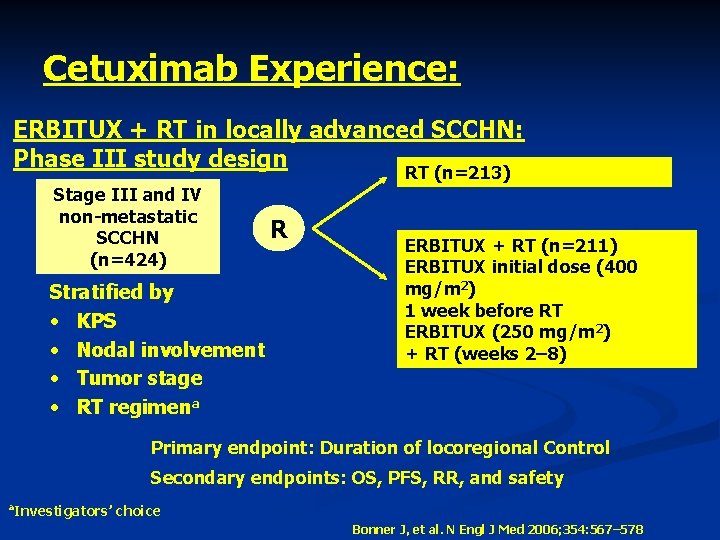
Cetuximab Experience: ERBITUX + RT in locally advanced SCCHN: Phase III study design RT (n=213) Stage III and IV non-metastatic SCCHN (n=424) Stratified by • KPS • Nodal involvement • Tumor stage • RT regimena R ERBITUX + RT (n=211) ERBITUX initial dose (400 mg/m 2) 1 week before RT ERBITUX (250 mg/m 2) + RT (weeks 2– 8) Primary endpoint: Duration of locoregional Control Secondary endpoints: OS, PFS, RR, and safety a Investigators’ choice Bonner J, et al. N Engl J Med 2006; 354: 567– 578

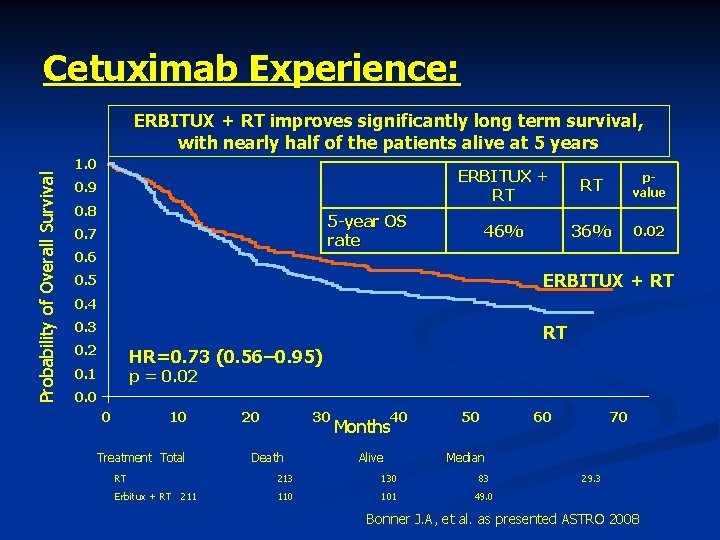
Cetuximab Experience: Probability of Overall Survival ERBITUX + RT improves significantly long term survival, with nearly half of the patients alive at 5 years 1. 0 0. 9 0. 8 ERBITUX + RT RT pvalue 46% 36% 0. 02 5 -year OS rate 0. 7 0. 6 ERBITUX + RT 0. 5 0. 4 0. 3 RT 0. 2 HR=0. 73 (0. 56– 0. 95) 0. 1 p = 0. 02 0. 0 0 10 Treatment Total 20 30 Death 40 Months Alive 50 60 70 Median RT 213 130 83 Erbitux + RT 211 110 101 49. 0 29. 3 Bonner J. A, et al. as presented ASTRO 2008

Bonner Trial Overview: n n n Significant Increase in Durability of Locoregional Control (HR = 0. 68, P = 0. 05). Better Median Duration for Locoregional Control (24. 4 vs 14. 9 months). Significant Reduction in Risk of Death (26%) (HR 0. 74, P = 0. 03). Independent Clinical Benefit. No Significant Increase in Grade 3 Co-morbid Events Apart From Acniform Rash & Fusion Reactions. No Significant Adverse Affection of Quality of Life. Incorporation of Molecularly Targeted Agents in The Primary Treatment of Squamous Cell Carcinoma of The Head & Neck. Jacques Bernier. Hematol Oncol Clin N Am. 22(2008)1193 -1208.

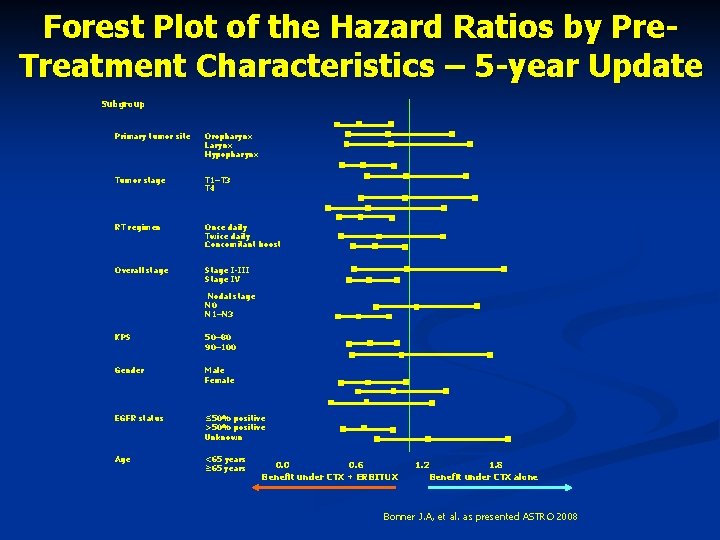
Forest Plot of the Hazard Ratios by Pre. Treatment Characteristics – 5 -year Update Subgroup Primary tumor site Oropharynx Larynx Hypopharynx Tumor stage T 1–T 3 T 4 RT regimen Once daily Twice daily Concomitant boost Overall stage Stage I-III Stage IV Nodal stage N 0 N 1–N 3 KPS 50– 80 90– 100 Gender Male Female EGFR status ≤ 50% positive >50% positive Unknown Age <65 years ≥ 65 years 0. 0 0. 6 Benefit under CTX + ERBITUX 1. 2 1. 8 Benefit under CTX alone Bonner J. A, et al. as presented ASTRO 2008

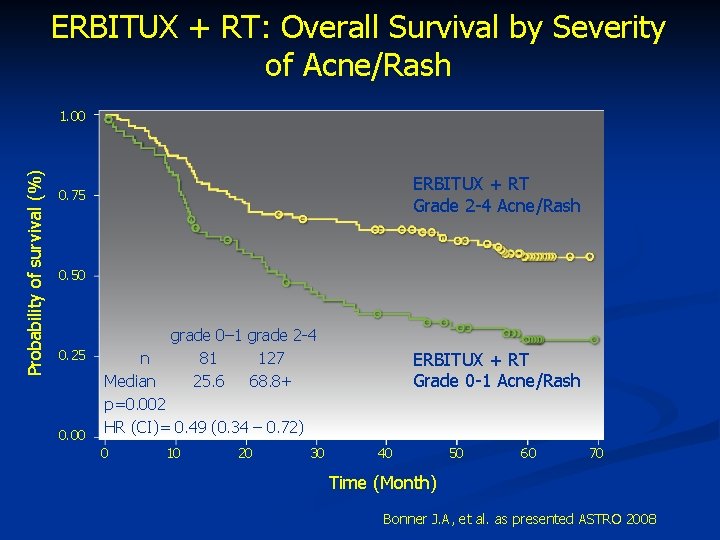
ERBITUX + RT: Overall Survival by Severity of Acne/Rash Probability of survival (%) 1. 00 ERBITUX + RT Grade 2 -4 Acne/Rash 0. 75 0. 50 0. 25 0. 00 grade 0– 1 grade 2 -4 n 81 127 Median 25. 6 68. 8+ p=0. 002 HR (CI)= 0. 49 (0. 34 – 0. 72) 0 10 20 30 ERBITUX + RT Grade 0 -1 Acne/Rash 40 50 60 70 Time (Month) Bonner J. A, et al. as presented ASTRO 2008

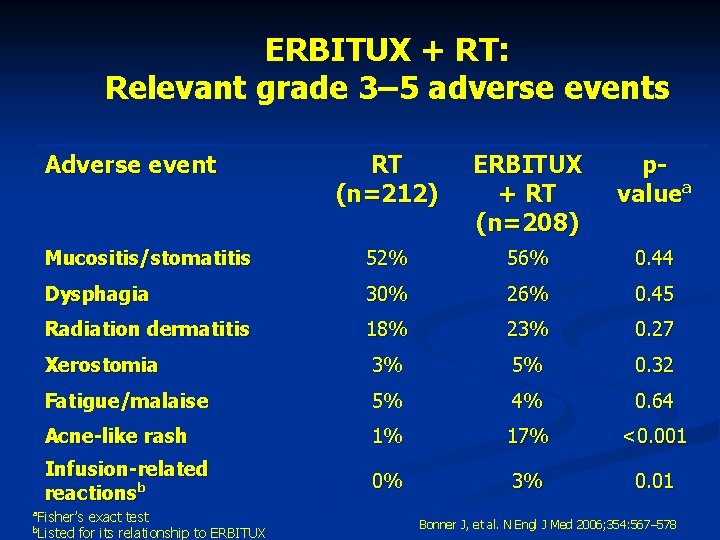
ERBITUX + RT: Relevant grade 3– 5 adverse events Adverse event a RT (n=212) ERBITUX + RT (n=208) pvaluea Mucositis/stomatitis 52% 56% 0. 44 Dysphagia 30% 26% 0. 45 Radiation dermatitis 18% 23% 0. 27 Xerostomia 3% 5% 0. 32 Fatigue/malaise 5% 4% 0. 64 Acne-like rash 1% 17% <0. 001 Infusion-related reactionsb 0% 3% 0. 01 Fisher’s exact test Listed for its relationship to ERBITUX b Bonner J, et al. N Engl J Med 2006; 354: 567– 578

Cetuximab + Rth CRT • No Phase III Direct Head to Head Comparison. • Between-Study Comparison of Phase III Studies 20 & 18 months Survival Advantages. • Discretion of The Treating Physician.

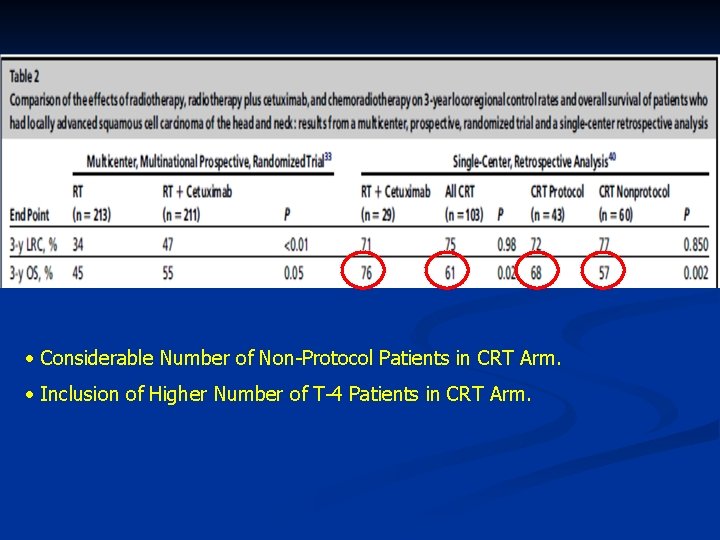
Cetuximab + Rth vs CRT? ? • Retrospective Analysis at ONE Center. • 29 Patients (Cetuximab + Rth) vs 103 Patients (CRT). Caudell JJ, Sawrie SM, Spencer SA, et al. Locoregionally advanced head and neck cancer treated with primary radiotherapy: a comparison of the addition of cetuximab or chemotherapy and the impact of protocol treatment. Int J Radiat Oncol Biol Phys 2008 [E-pub]. Item Cetuximab + Rth CRT P-Value 3 -Y L. C. 71% 75% NS Distant Metastases FS 92% 87% NS Disease Specific Survival 79% 77% NS 3 -Y OAS 76% 61% 0. 02

• Considerable Number of Non-Protocol Patients in CRT Arm. • Inclusion of Higher Number of T-4 Patients in CRT Arm.

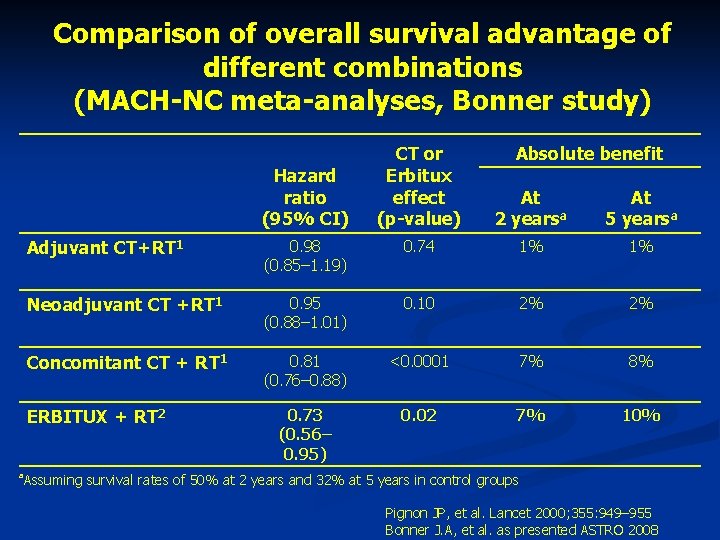
Comparison of overall survival advantage of different combinations (MACH-NC meta-analyses, Bonner study) Hazard ratio (95% CI) At 2 yearsa At 5 yearsa Adjuvant CT+RT 1 0. 98 (0. 85– 1. 19) 0. 74 1% 1% Neoadjuvant CT +RT 1 0. 95 (0. 88– 1. 01) 0. 10 2% 2% Concomitant CT + RT 1 0. 81 (0. 76– 0. 88) <0. 0001 7% 8% 0. 73 (0. 56– 0. 95) 0. 02 7% 10% ERBITUX + RT 2 a Absolute benefit CT or Erbitux effect (p-value) Assuming survival rates of 50% at 2 years and 32% at 5 years in control groups Pignon JP, et al. Lancet 2000; 355: 949– 955 Bonner J. A, et al. as presented ASTRO 2008

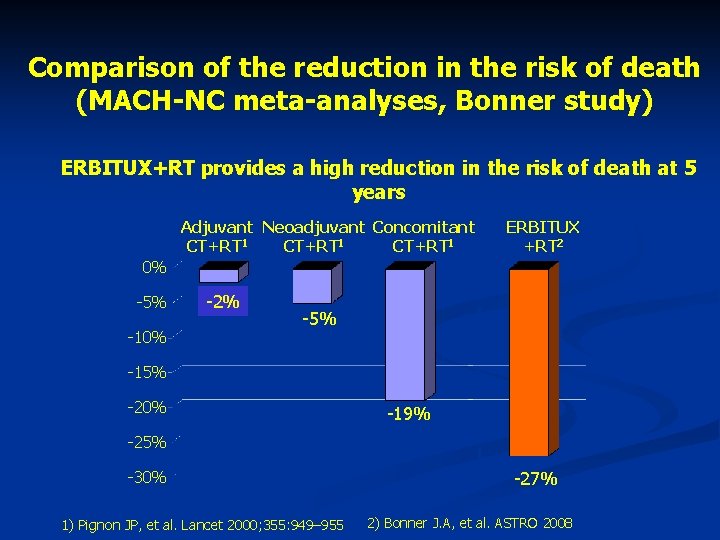
Comparison of the reduction in the risk of death (MACH-NC meta-analyses, Bonner study) ERBITUX+RT provides a high reduction in the risk of death at 5 years Adjuvant Neoadjuvant Concomitant CT+RT 1 ERBITUX +RT 2 0% -5% -10% -2% -5% -15% -20% -19% -25% -30% 1) Pignon JP, et al. Lancet 2000; 355: 949– 955 -27% 2) Bonner J. A, et al. ASTRO 2008

Cetuximab + CRT in Phase III Trials in Advanced HNSCC: n n Radiation Therapy Oncology Group: Cisplatin-Based CRT +/- Cetuximab. Groupe Oncologie Radiotherapie Tet et Cou: Rth + Cetuximab vs Cetuximab + Carboplatin/5 -Fu-Based CRT. Pfister DG, Su YB, Kraus DH, et al. Concurrent cetuximab, cisplatin, and concomitant boost radiotherapy for locoregionally advanced, squamous cell head and neck cancer: a pilot phase III study of a new combined-modality paradigm. J Clin Oncol 2006; 24(7): 1072– 8

Other Epidermal Growth Factor Receptor. Targeted Monoclonal Antibodies Phase I/II: n n n Panitumumab (Vectibix). Zalutumumab (Humax-EGFr). Nimotuzumab (Theraloc). Epidermal Growth Factor Tyrosin Kinase Inhibitors Phase I/II Trials: n n Gefitinib (Iressa) + Cisplatin + Accelerated Rth: CR in 52% (46 Patients). Erlotinib (Tarceva) + Cisplatin-Based CRT: CR in 84% (25 Patients).

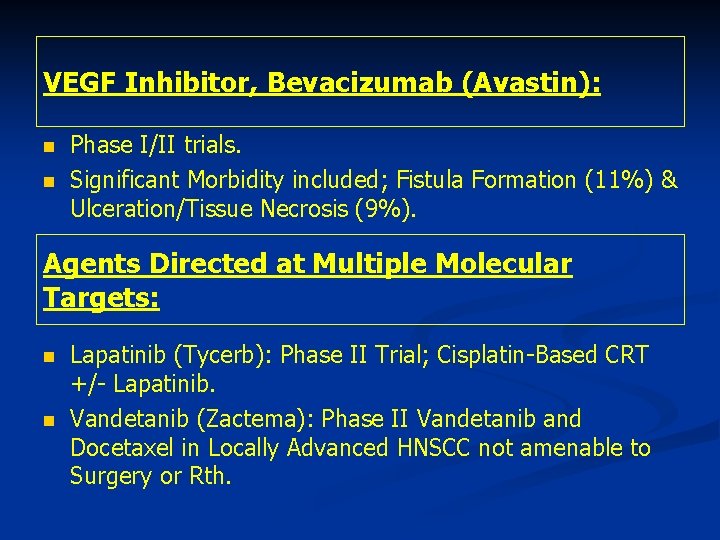
VEGF Inhibitor, Bevacizumab (Avastin): n n Phase I/II trials. Significant Morbidity included; Fistula Formation (11%) & Ulceration/Tissue Necrosis (9%). Agents Directed at Multiple Molecular Targets: n n Lapatinib (Tycerb): Phase II Trial; Cisplatin-Based CRT +/- Lapatinib. Vandetanib (Zactema): Phase II Vandetanib and Docetaxel in Locally Advanced HNSCC not amenable to Surgery or Rth.
 Squamous cell carcinoma louisiana
Squamous cell carcinoma louisiana Squamous cell carcinoma
Squamous cell carcinoma Non neoplastic epithelial disorders
Non neoplastic epithelial disorders Hedgehog urchin
Hedgehog urchin Papillary carcinoma
Papillary carcinoma Naphthylamine
Naphthylamine Icd 10 tumor gaster
Icd 10 tumor gaster Basal cell carcinoma
Basal cell carcinoma Basal cell carcinoma
Basal cell carcinoma Malignancy
Malignancy Fractuer
Fractuer My ukulele has a body a neck and a head
My ukulele has a body a neck and a head Tnm 8 head and neck
Tnm 8 head and neck Risk factors of head and neck cancer
Risk factors of head and neck cancer Thumb brush strum
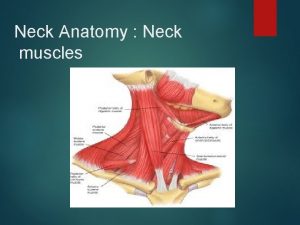
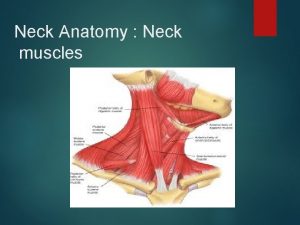
Thumb brush strum Muscular system head and neck
Muscular system head and neck Muscles of the head neck and shoulders
Muscles of the head neck and shoulders Regional write up head face and neck
Regional write up head face and neck What has a neck but no head
What has a neck but no head Slidetodoc.com
Slidetodoc.com Carcinoma in situ
Carcinoma in situ Neoplasia
Neoplasia Invasive ductal carcinoma with medullary features
Invasive ductal carcinoma with medullary features Tnm breast cancer staging
Tnm breast cancer staging Carcinoma de mama
Carcinoma de mama Carcinoma in situ
Carcinoma in situ Breast cancer biopsy
Breast cancer biopsy Radiologix verdun
Radiologix verdun Follicular carcinoma of thyroid
Follicular carcinoma of thyroid Breast papillary carcinoma
Breast papillary carcinoma Hormones
Hormones Carcinoma micropapilar invasivo de mama
Carcinoma micropapilar invasivo de mama