Lecture 13 review nephron structure significance to lipid


- Slides: 29

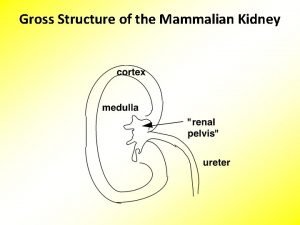
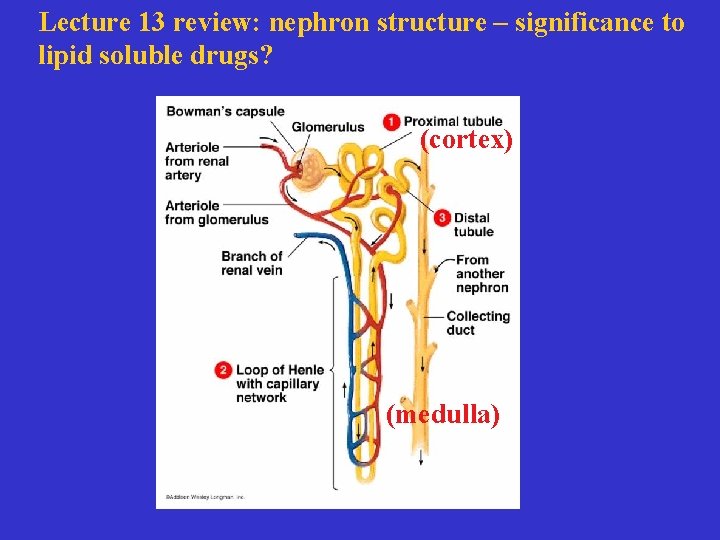
Lecture 13 review: nephron structure – significance to lipid soluble drugs? (cortex) (medulla)

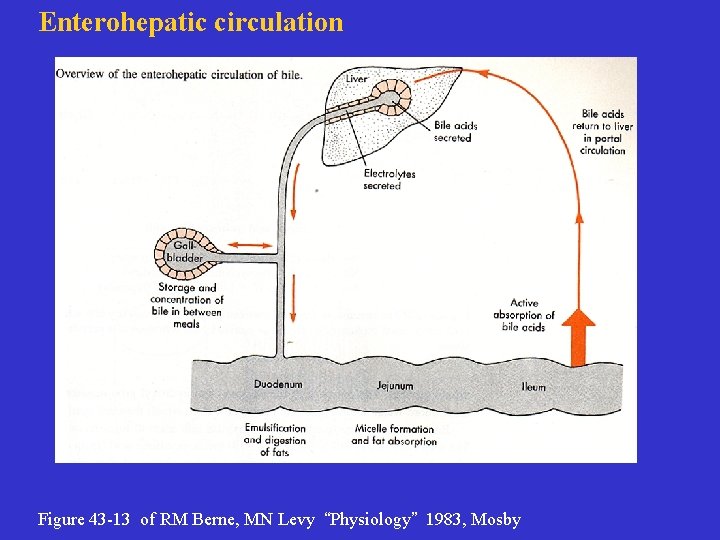
Enterohepatic circulation Figure 43 -13 of RM Berne, MN Levy “Physiology” 1983, Mosby

Will imatinib last forever in the body? P=24, 000

Lecture 14: Drug biotransformation (Chapter 8 of Levine’s Pharmacology) Important definitions: 1. biotransformation vs metabolism 1. xenobiotics Q 1: Why do we have enzymes that metabolize/ biotransform drugs? What is the evolutionary rationale for their presence?

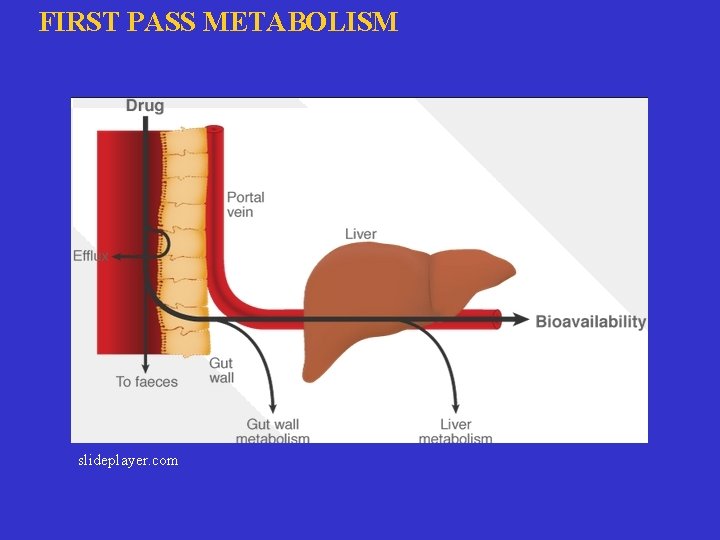
FIRST PASS METABOLISM slideplayer. com

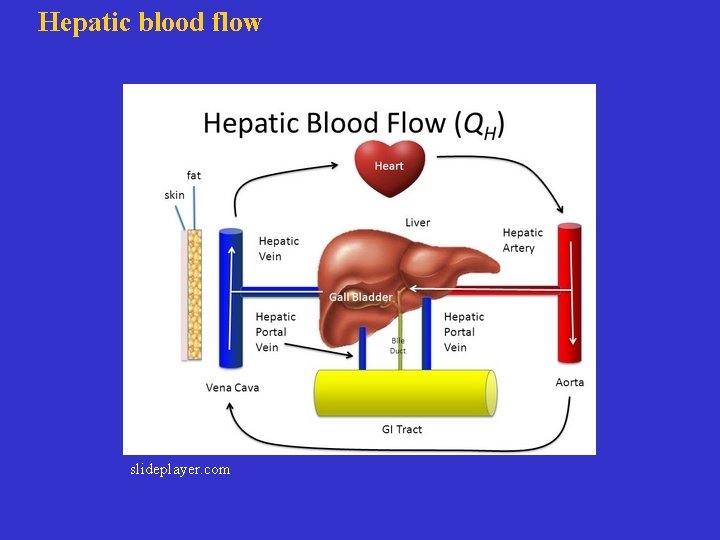
Hepatic blood flow slideplayer. com

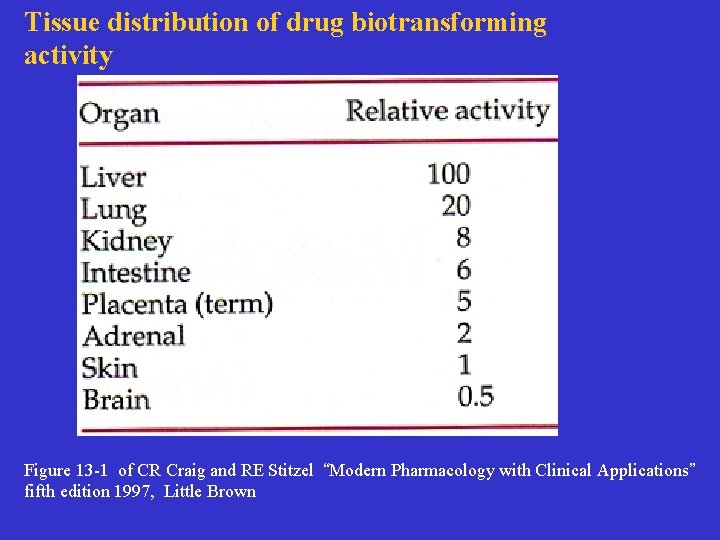
Tissue distribution of drug biotransforming activity Figure 13 -1 of CR Craig and RE Stitzel “Modern Pharmacology with Clinical Applications” fifth edition 1997, Little Brown

Q: What does an organic chemist look for in evaluating the potential reactivity of a molecule?

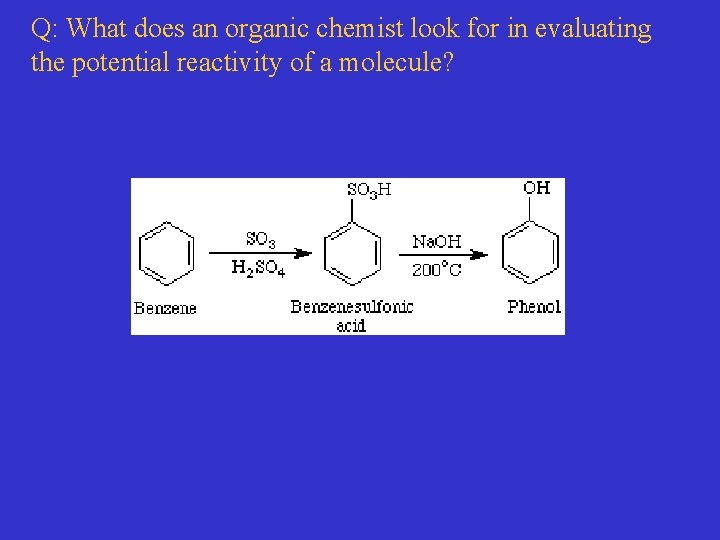
Q: What does an organic chemist look for in evaluating the potential reactivity of a molecule?

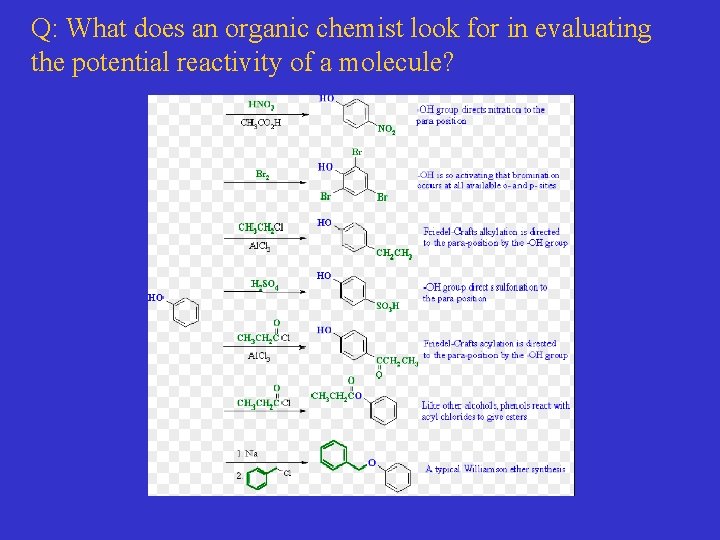
Q: What does an organic chemist look for in evaluating the potential reactivity of a molecule?

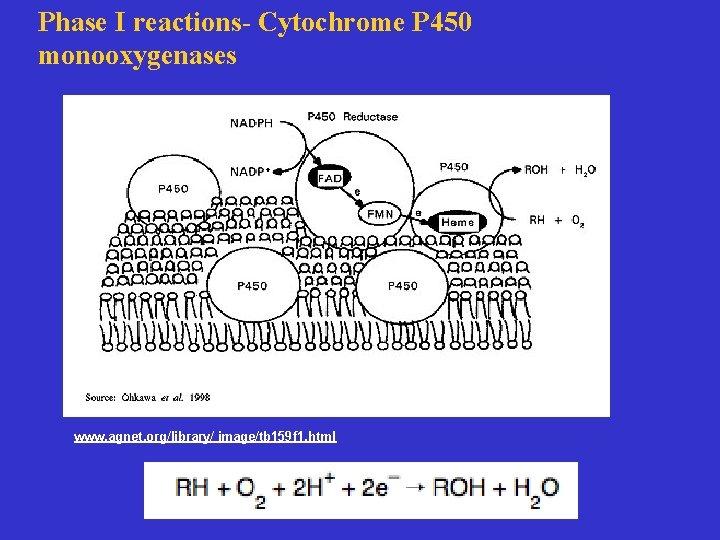
Phase I reactions- Cytochrome P 450 monooxygenases www. agnet. org/library/ image/tb 159 f 1. html

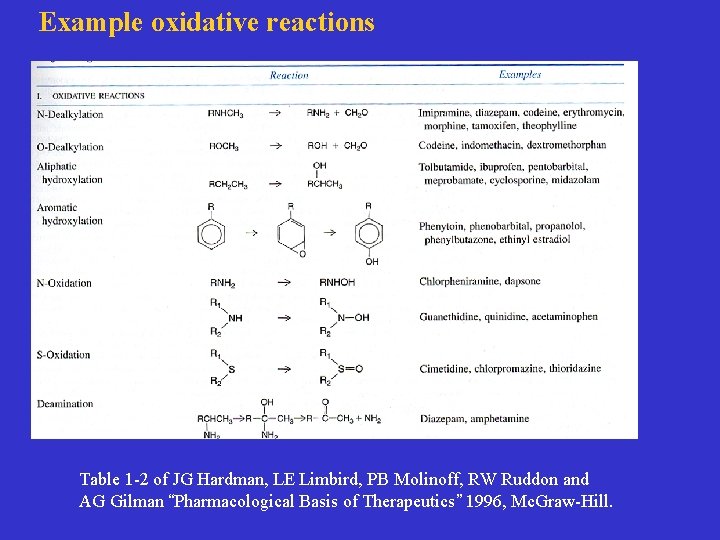
Example oxidative reactions Table 1 -2 of JG Hardman, LE Limbird, PB Molinoff, RW Ruddon and AG Gilman “Pharmacological Basis of Therapeutics” 1996, Mc. Graw-Hill.

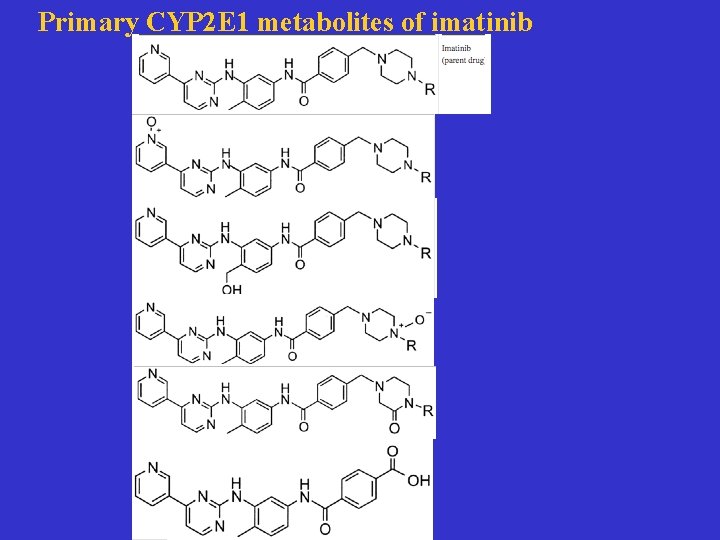
Primary CYP 2 E 1 metabolites of imatinib

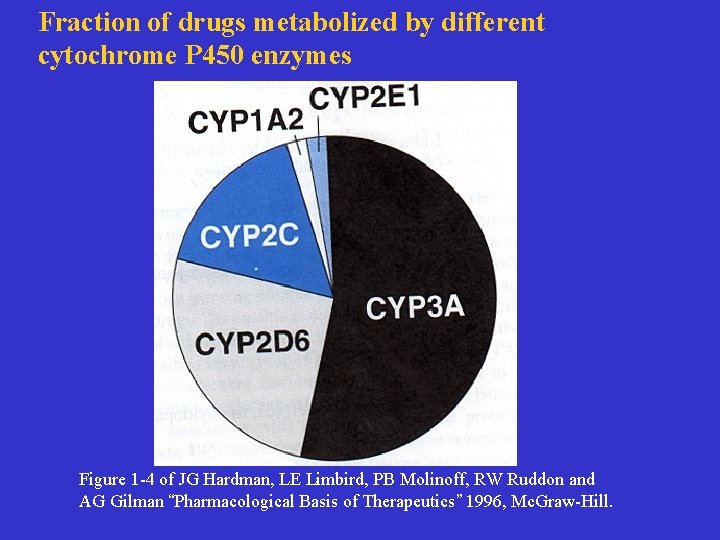
Fraction of drugs metabolized by different cytochrome P 450 enzymes Figure 1 -4 of JG Hardman, LE Limbird, PB Molinoff, RW Ruddon and AG Gilman “Pharmacological Basis of Therapeutics” 1996, Mc. Graw-Hill.

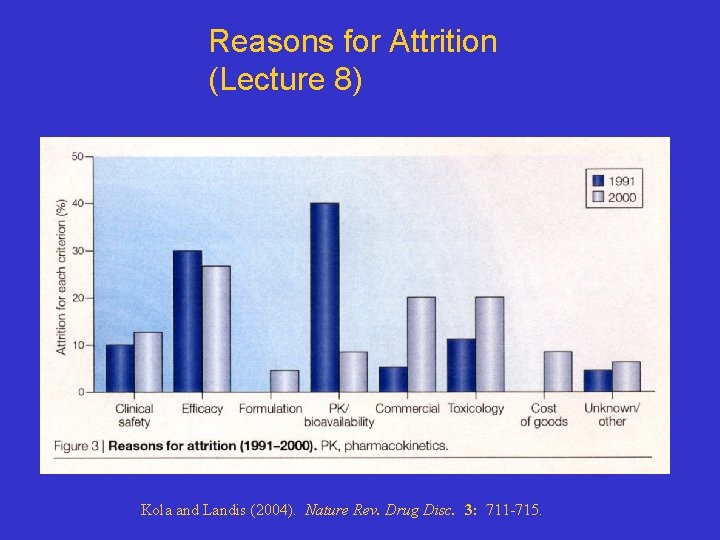
Reasons for Attrition (Lecture 8) Kola and Landis (2004). Nature Rev. Drug Disc. 3: 711 -715.

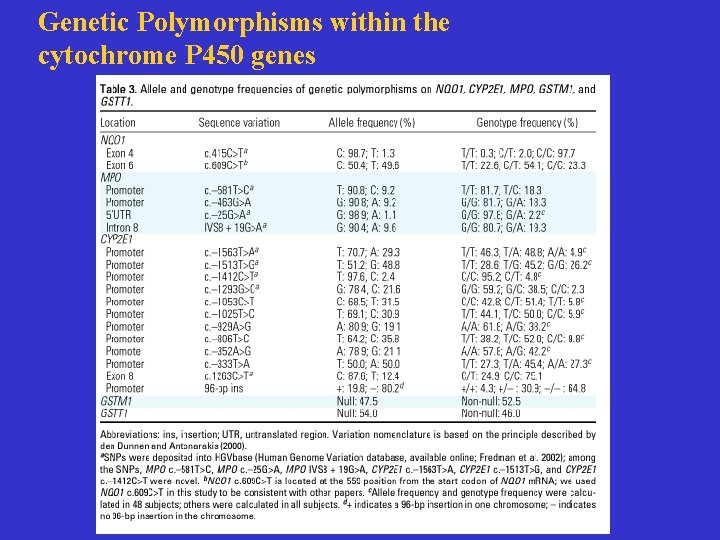
Genetic Polymorphisms within the cytochrome P 450 genes

Q: What could happen if two different drugs were administered that are acted upon by the same CYP 450 enzyme?

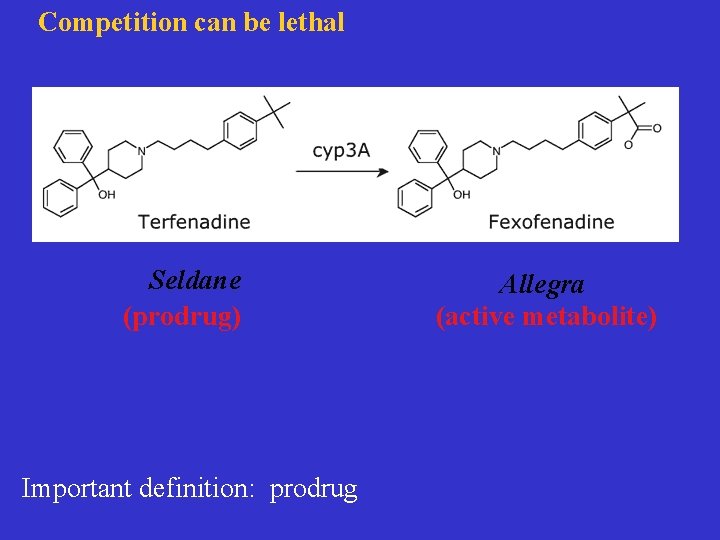
Competition can be lethal Seldane (prodrug) Important definition: prodrug Allegra (active metabolite)

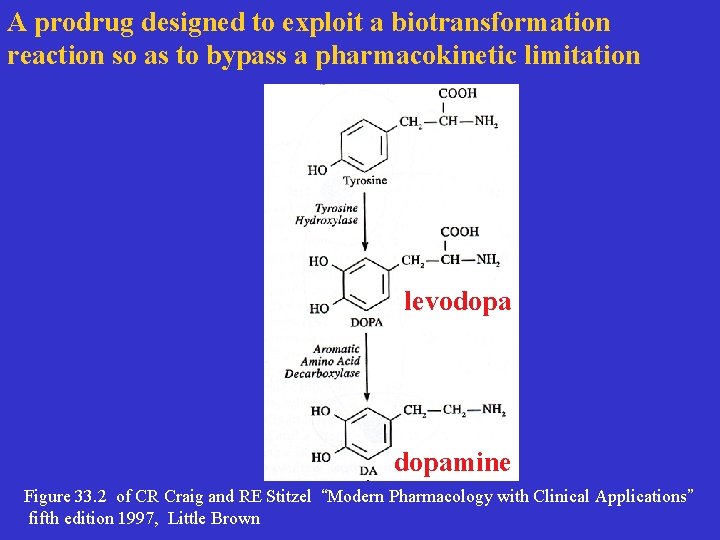
A prodrug designed to exploit a biotransformation reaction so as to bypass a pharmacokinetic limitation levodopamine Figure 33. 2 of CR Craig and RE Stitzel “Modern Pharmacology with Clinical Applications” fifth edition 1997, Little Brown

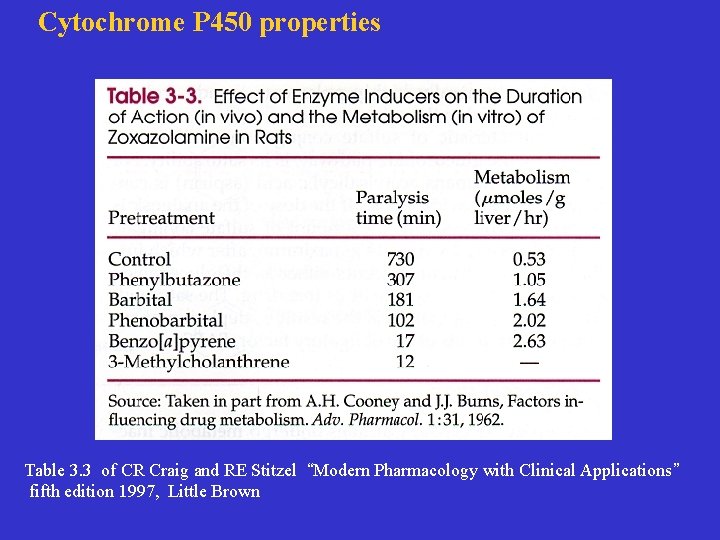
Cytochrome P 450 properties Table 3. 3 of CR Craig and RE Stitzel “Modern Pharmacology with Clinical Applications” fifth edition 1997, Little Brown

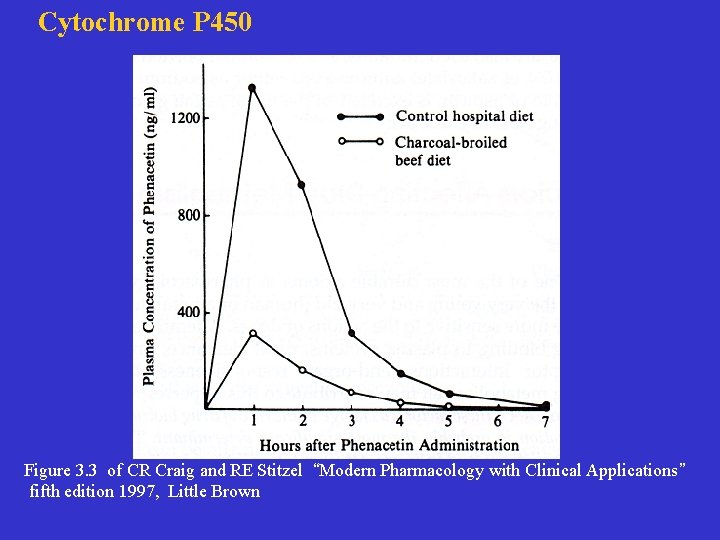
Cytochrome P 450 Figure 3. 3 of CR Craig and RE Stitzel “Modern Pharmacology with Clinical Applications” fifth edition 1997, Little Brown

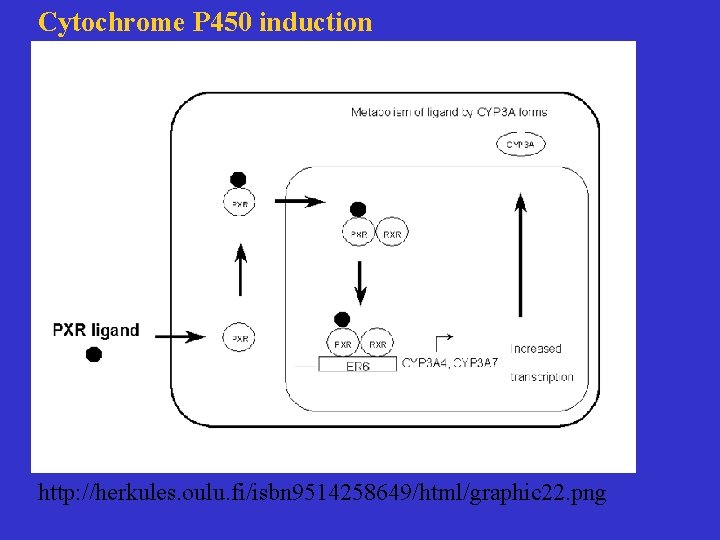
Cytochrome P 450 induction http: //herkules. oulu. fi/isbn 9514258649/html/graphic 22. png

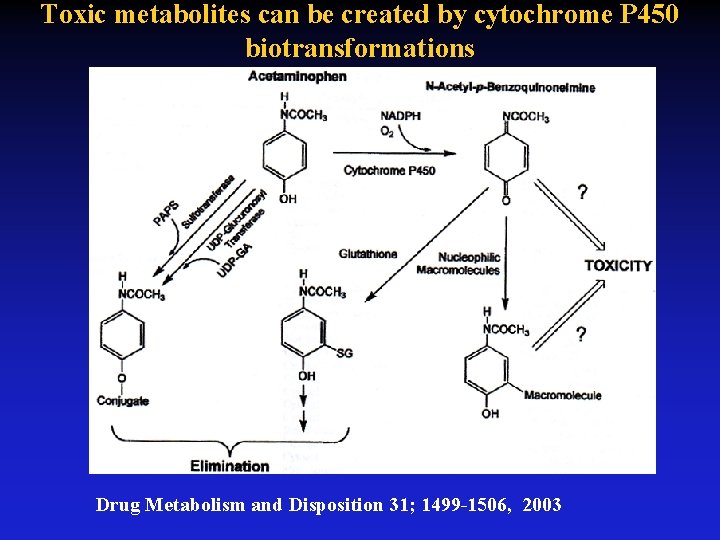
Toxic metabolites can be created by cytochrome P 450 biotransformations Drug Metabolism and Disposition 31; 1499 -1506, 2003

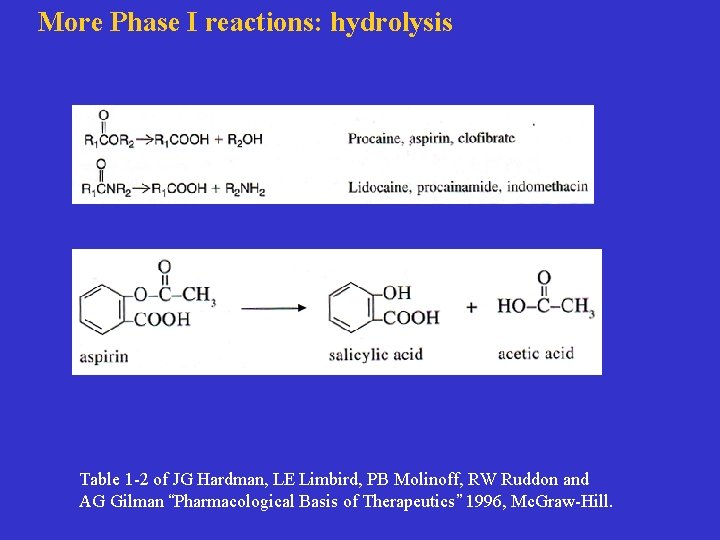
More Phase I reactions: hydrolysis Table 1 -2 of JG Hardman, LE Limbird, PB Molinoff, RW Ruddon and AG Gilman “Pharmacological Basis of Therapeutics” 1996, Mc. Graw-Hill.

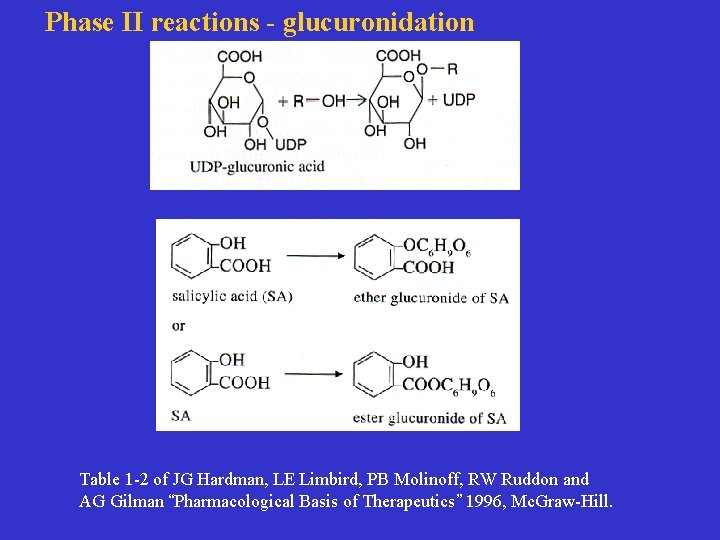
Phase II reactions - glucuronidation Table 1 -2 of JG Hardman, LE Limbird, PB Molinoff, RW Ruddon and AG Gilman “Pharmacological Basis of Therapeutics” 1996, Mc. Graw-Hill.

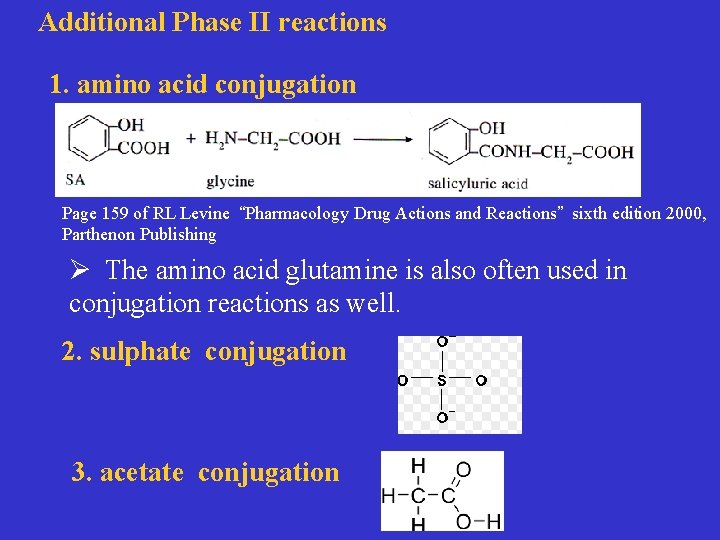
Additional Phase II reactions 1. amino acid conjugation Page 159 of RL Levine “Pharmacology Drug Actions and Reactions” sixth edition 2000, Parthenon Publishing Ø The amino acid glutamine is also often used in conjugation reactions as well. 2. sulphate conjugation 3. acetate conjugation

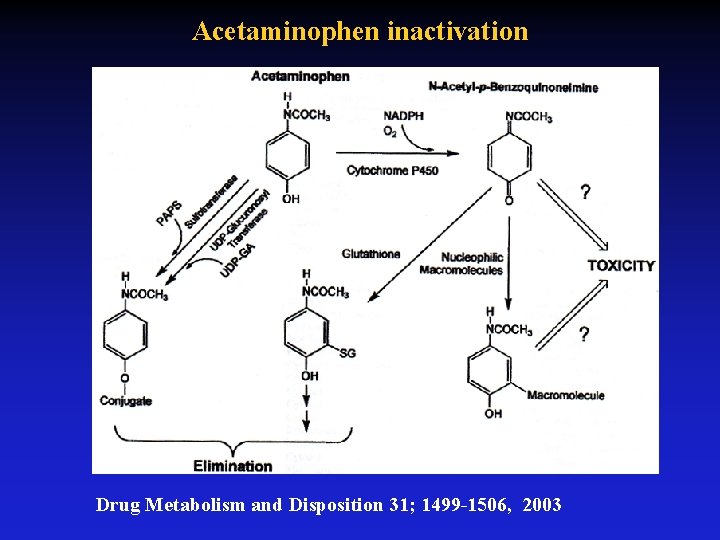
Acetaminophen inactivation Drug Metabolism and Disposition 31; 1499 -1506, 2003

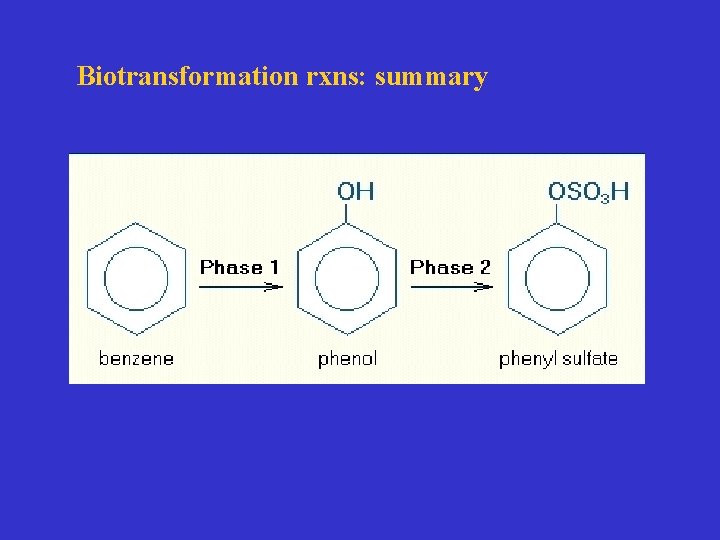
Biotransformation rxns: summary

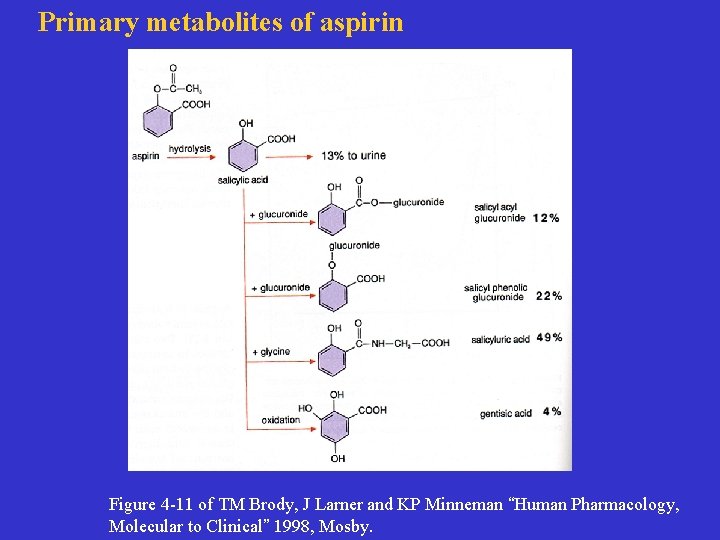
Primary metabolites of aspirin Figure 4 -11 of TM Brody, J Larner and KP Minneman “Human Pharmacology, Molecular to Clinical” 1998, Mosby.
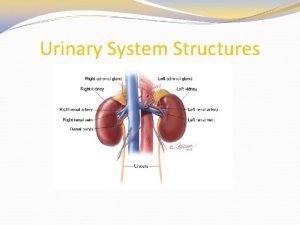
 Figure 15-2 is a longitudinal section of a kidney
Figure 15-2 is a longitudinal section of a kidney Adh act on
Adh act on 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad Cellulose amylose amylopectin and glycogen
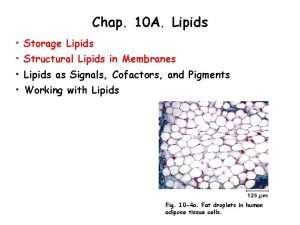
Cellulose amylose amylopectin and glycogen Structure of storage lipids
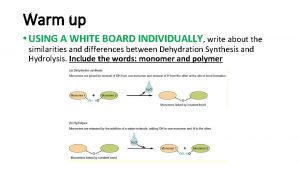
Structure of storage lipids Lipid structure
Lipid structure Lipid structure
Lipid structure Principal cells
Principal cells Figure 15-3 is a diagram of the nephron
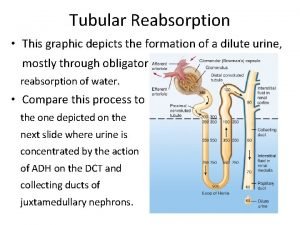
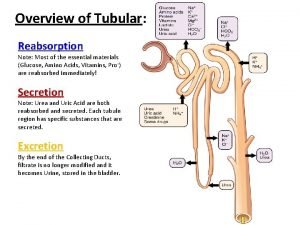
Figure 15-3 is a diagram of the nephron Tubule reabsorption
Tubule reabsorption Juxtamedullary nephron
Juxtamedullary nephron Efron on the nephron
Efron on the nephron Where does secretion occur in nephron
Where does secretion occur in nephron Transcellular
Transcellular Reabsorpsi
Reabsorpsi Distal tubule
Distal tubule Reabsorption in the nephron occurs in the
Reabsorption in the nephron occurs in the Tubuloglomerular feedback mechanism
Tubuloglomerular feedback mechanism Figure 15-4 is a diagram of a nephron
Figure 15-4 is a diagram of a nephron Tubuloglomerular feedback
Tubuloglomerular feedback Urine rm
Urine rm Catheter bag
Catheter bag Youtube urinary system
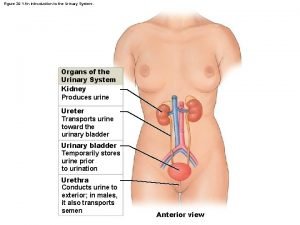
Youtube urinary system Figure 15-3 the urinary system
Figure 15-3 the urinary system Distal convoluted tubule
Distal convoluted tubule What is the name
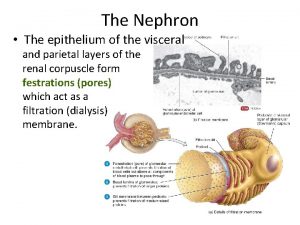
What is the name Renal corpuscle
Renal corpuscle Cummings
Cummings Bowman's capsule
Bowman's capsule Nephron
Nephron