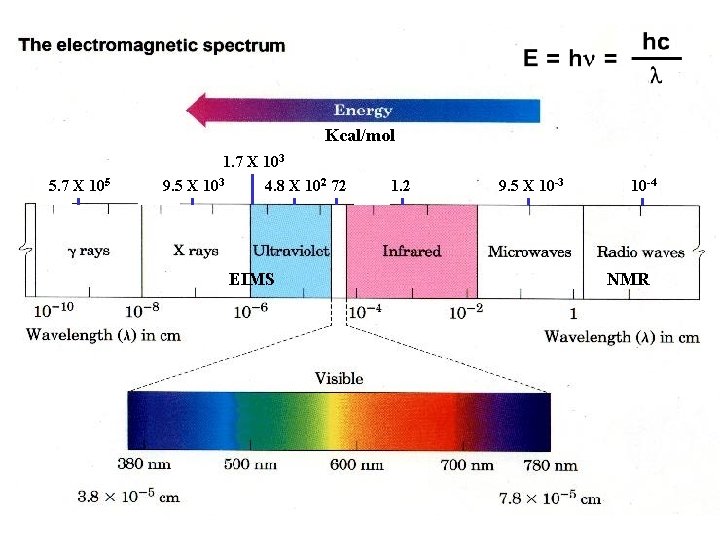
Kcalmol 5 7 X 105 1 7 X












- Slides: 51

Kcal/mol 5. 7 X 105 1. 7 X 103 9. 5 X 103 4. 8 X 102 72 EIMS 1. 2 9. 5 X 10 -3 10 -4 NMR

Nuclear Magnetic Resonance (NMR) Spectroscopy From here… To here!

The Nobel Prize in Physics 1952 "for their development of new methods for nuclear magnetic precision measurements and discoveries in connection therewith " Felix Bloch Edward Mills Purcell

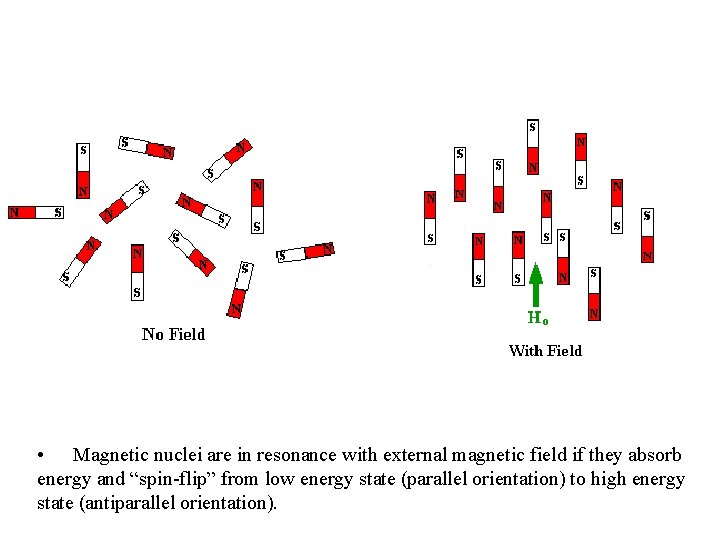
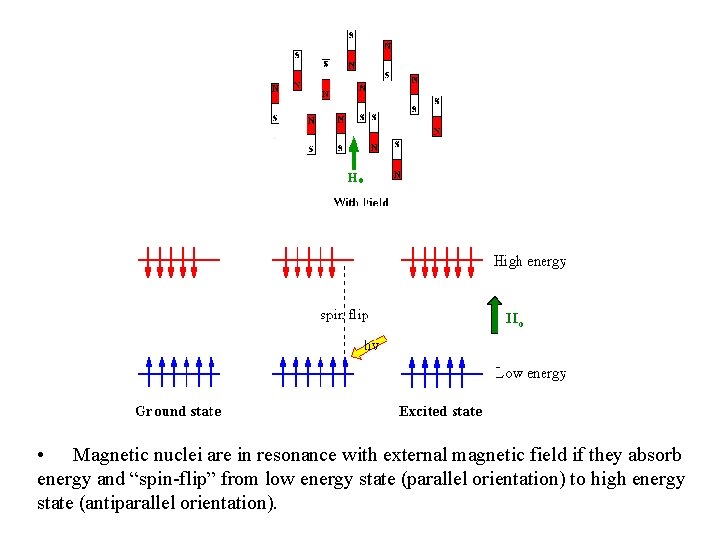
• Magnetic nuclei are in resonance with external magnetic field if they absorb energy and “spin-flip” from low energy state (parallel orientation) to high energy state (antiparallel orientation).

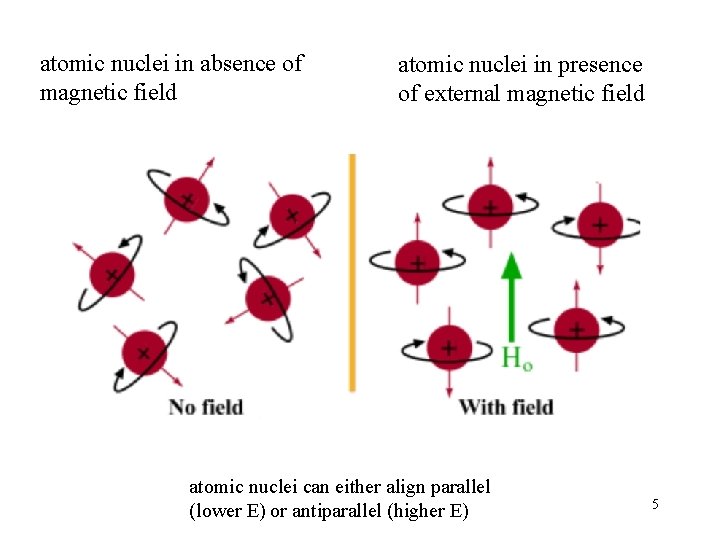
atomic nuclei in absence of magnetic field atomic nuclei in presence of external magnetic field atomic nuclei can either align parallel (lower E) or antiparallel (higher E) 5

• Magnetic nuclei are in resonance with external magnetic field if they absorb energy and “spin-flip” from low energy state (parallel orientation) to high energy state (antiparallel orientation).

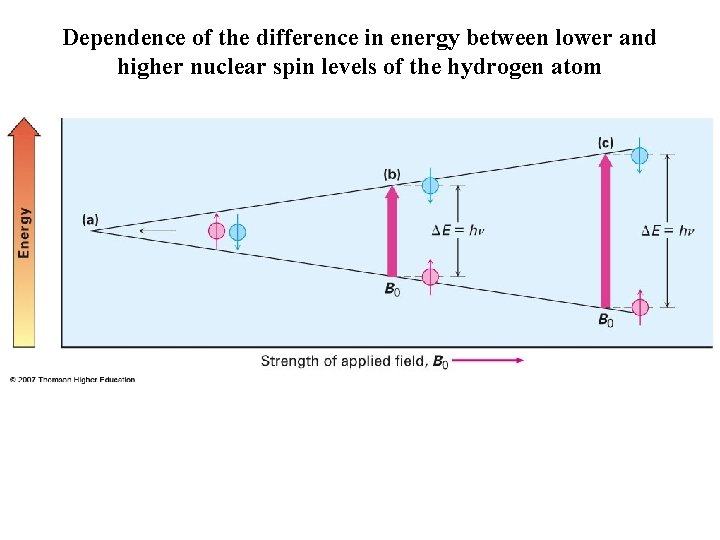
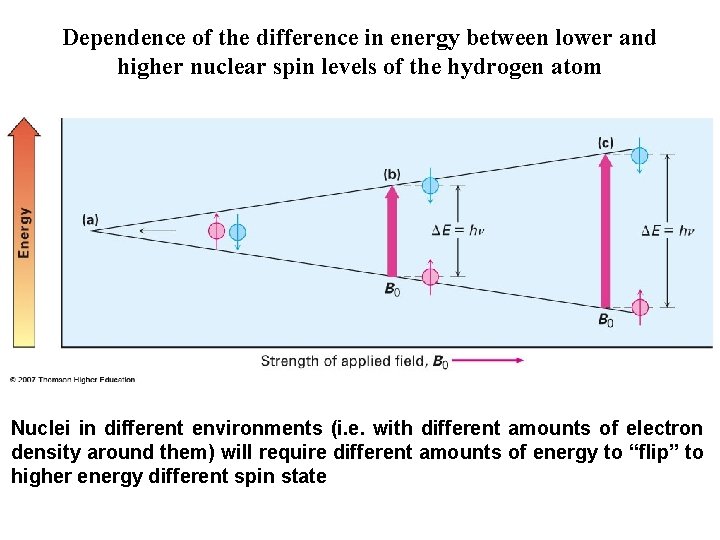
Dependence of the difference in energy between lower and higher nuclear spin levels of the hydrogen atom

Dependence of the difference in energy between lower and higher nuclear spin levels of the hydrogen atom Nuclei in different environments (i. e. with different amounts of electron density around them) will require different amounts of energy to “flip” to higher energy different spin state

Magnetic: o All nuclei with odd number of protons o All nuclei with odd number of neutrons Nonmagnetic: o Nuclei with even number of both protons and neutrons 9

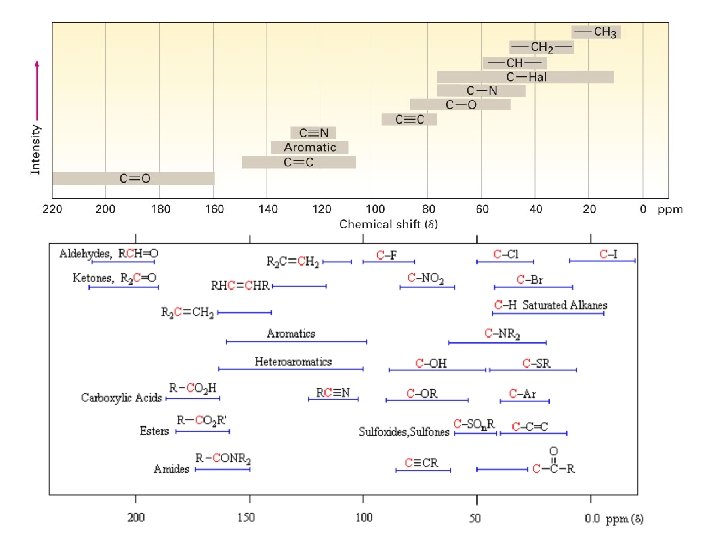
Fig. 13 -4, p. 444

11

Really Old School: Continuous wave (CW) 40 MHz NMR spectrometer 1960

A little less old school: Continuous wave (CW) 60 MHz NMR spectrum 1964

Not quite so old school: 1980’s 60 MHz

The Nobel Prize in Chemistry 1991 "for his contributions to the development of the methodology of high resolution nuclear magnetic resonance (NMR) spectroscopy" Richard R. Ernst

State-of-the-art 900 MHz NMR spectrometer Center for Biomolecular NMR, Heinrich-Heine-Universität Düsseldorf

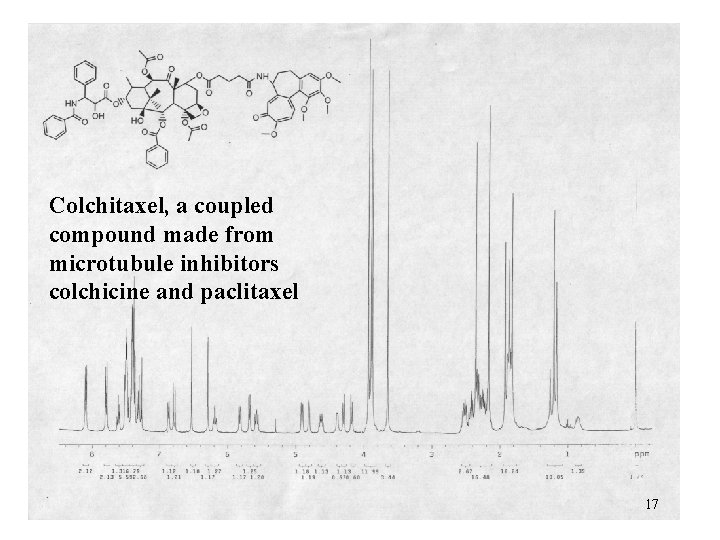
Colchitaxel, a coupled compound made from microtubule inhibitors colchicine and paclitaxel 17

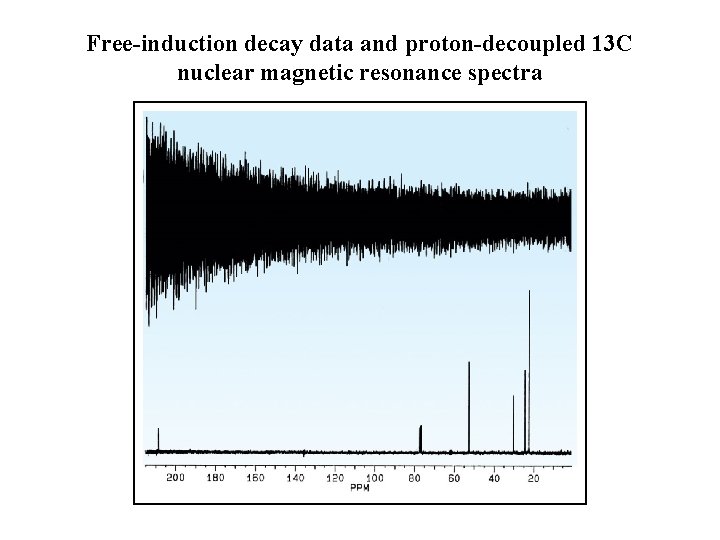
Free-induction decay data and proton-decoupled 13 C nuclear magnetic resonance spectra

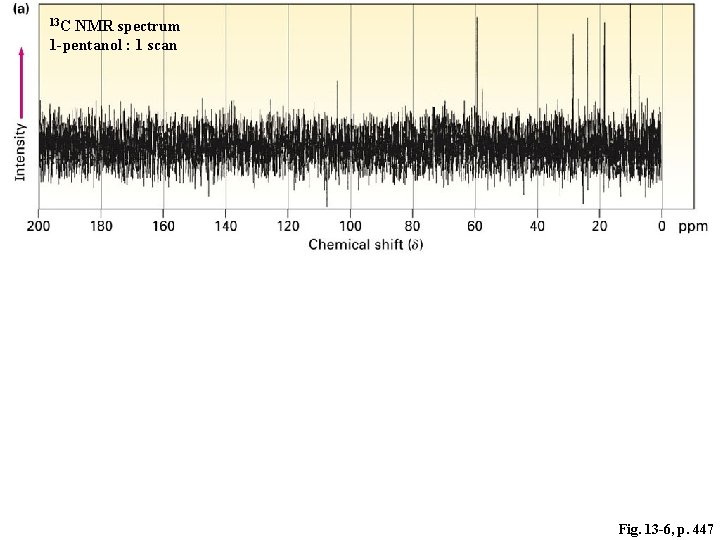
13 C NMR spectrum 1 -pentanol : 1 scan Fig. 13 -6, p. 447

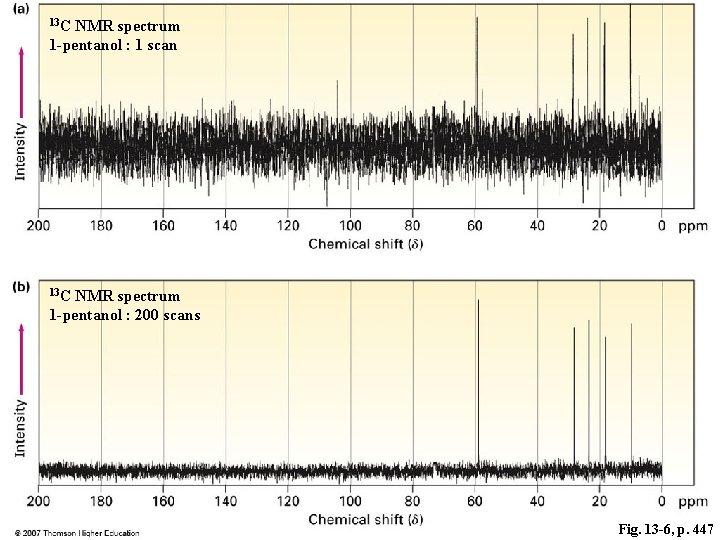
13 C NMR spectrum 1 -pentanol : 1 scan 13 C NMR spectrum 1 -pentanol : 200 scans Fig. 13 -6, p. 447

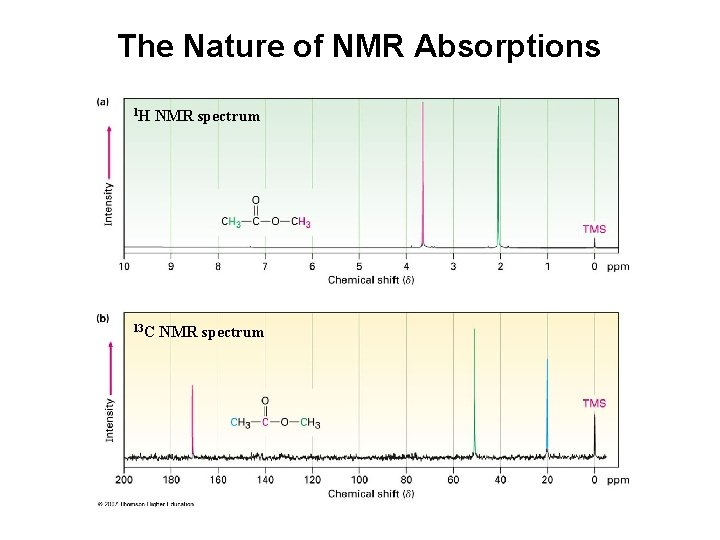
The Nature of NMR Absorptions 1 H NMR spectrum 13 C NMR spectrum

The Nobel Prize in Chemistry 2002 "for his development of nuclear magnetic resonance spectroscopy for determining the three-dimensional structure of biological macromolecules in solution" Kurt Wüthrich

The Nobel Prize in Medicine 2003 "for their discoveries concerning magnetic resonance imaging " Paul C. Lauterbur Sir Peter Mansfield

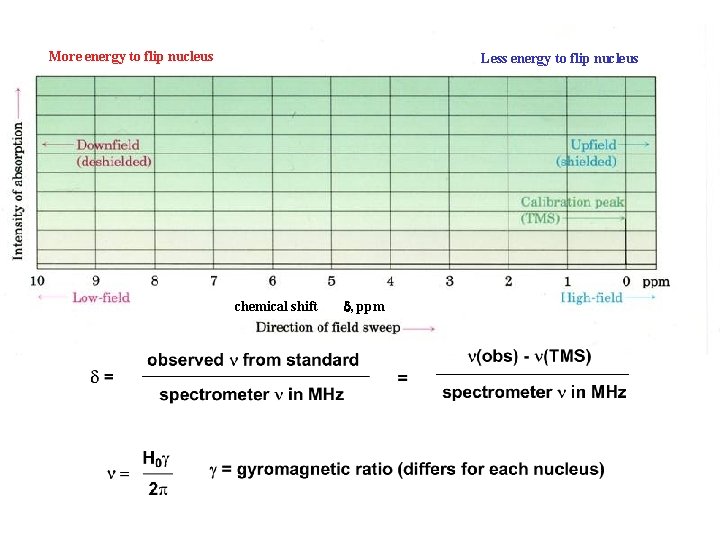
More energy to flip nucleus Less energy to flip nucleus chemical shift d, ppm

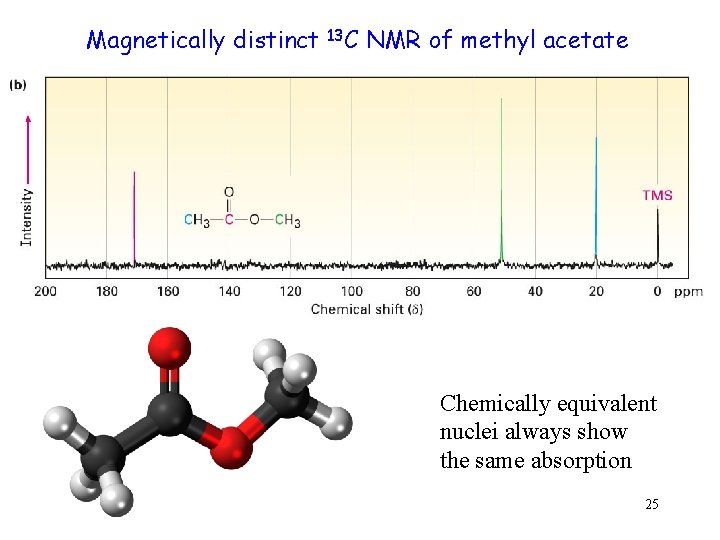
Magnetically distinct 13 C NMR of methyl acetate Chemically equivalent nuclei always show the same absorption 25

Magnetically distinct hydrogens and carbons! 26

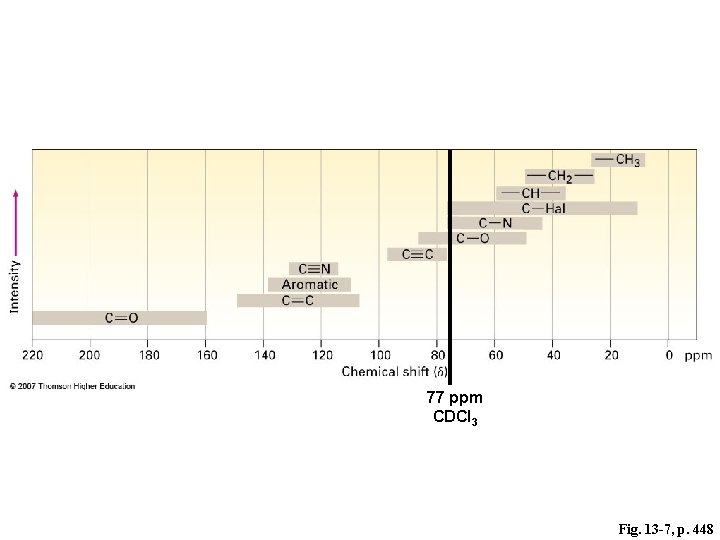
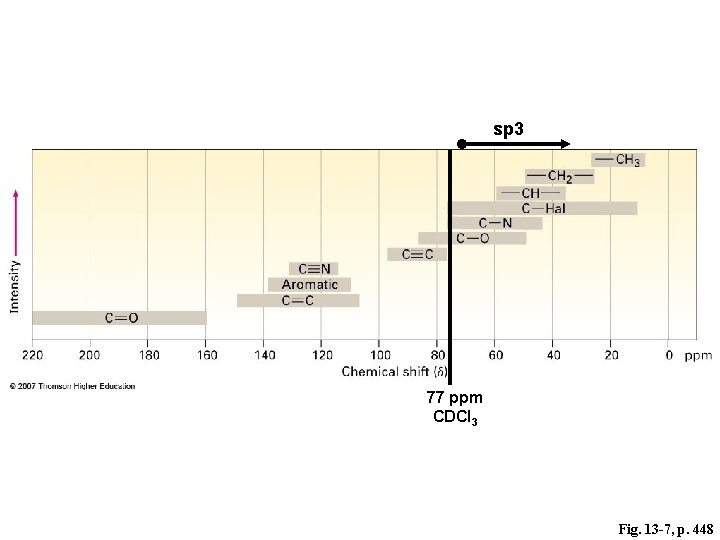
77 ppm CDCl 3 Fig. 13 -7, p. 448

sp 3 77 ppm CDCl 3 Fig. 13 -7, p. 448

29


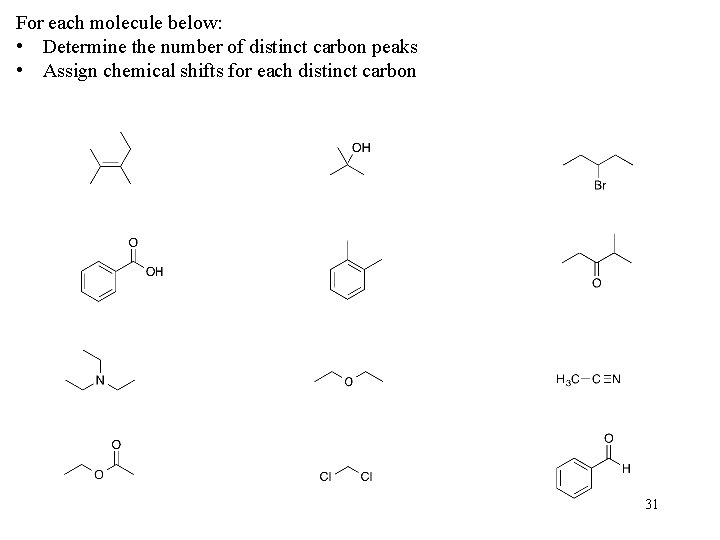
For each molecule below: • Determine the number of distinct carbon peaks • Assign chemical shifts for each distinct carbon 31

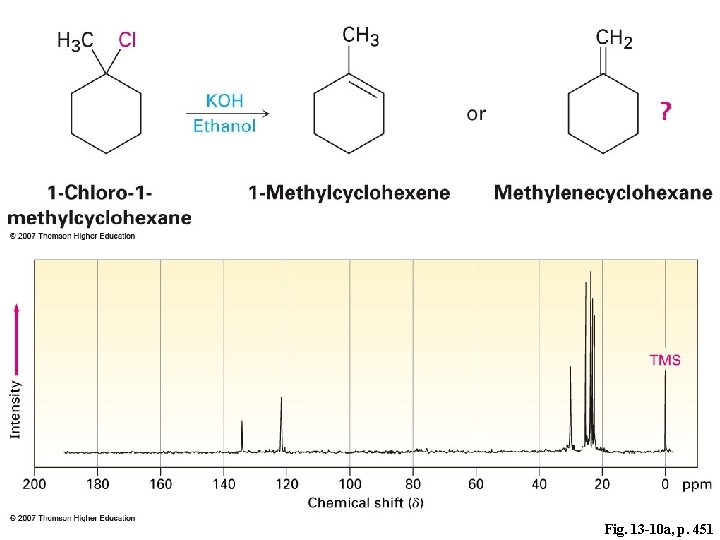
Fig. 13 -10 a, p. 451

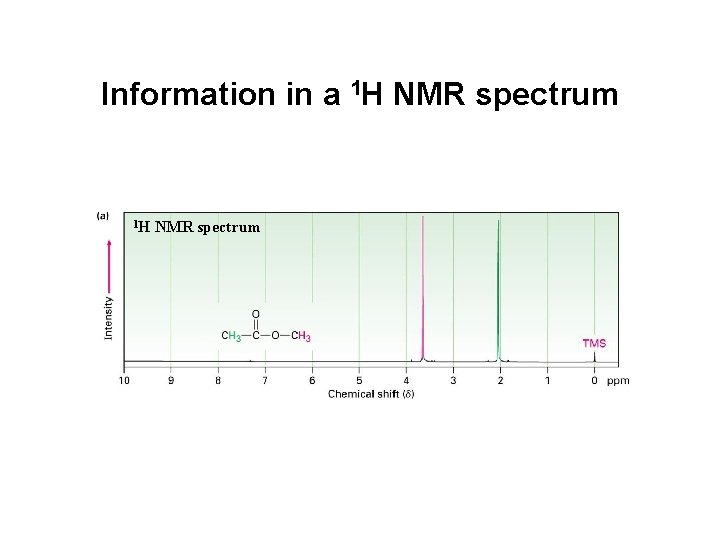
Information in a 1 H NMR spectrum 13 C NMR spectrum

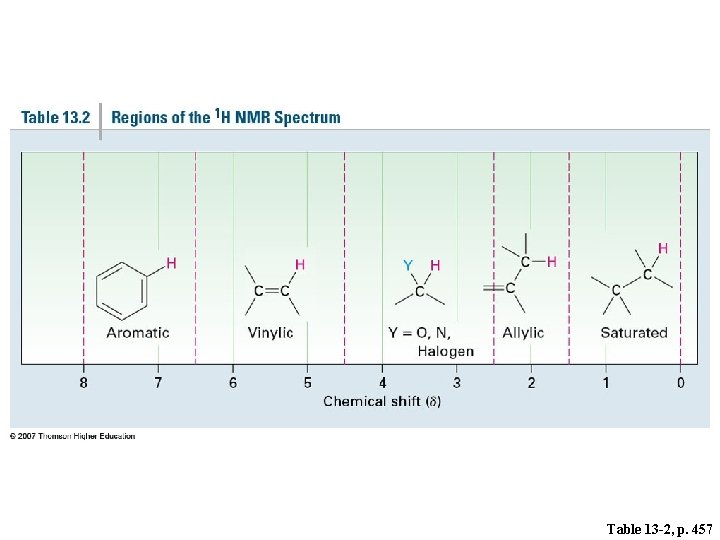
Table 13 -2, p. 457

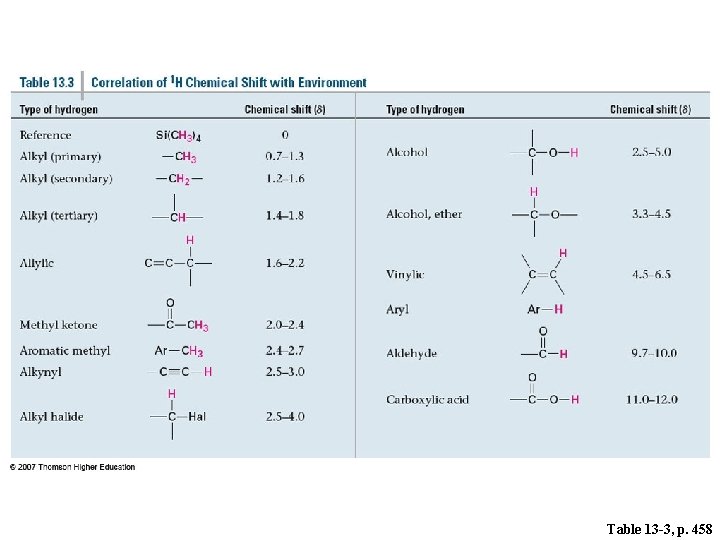
Table 13 -3, p. 458

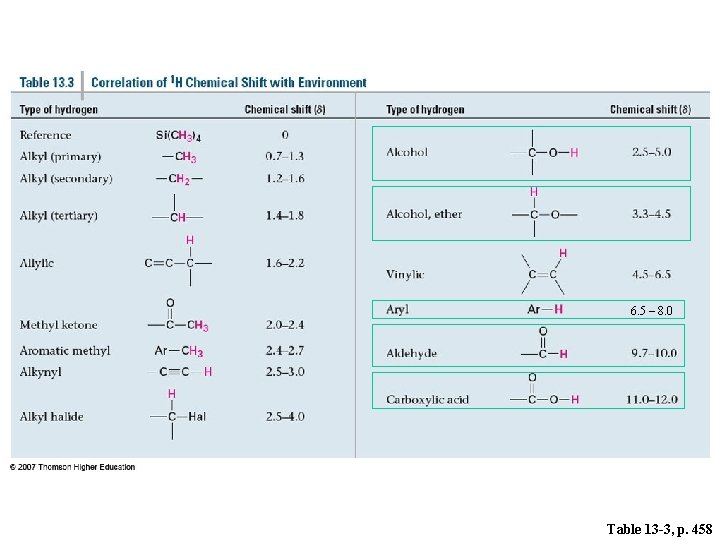
6. 5 – 8. 0 Table 13 -3, p. 458

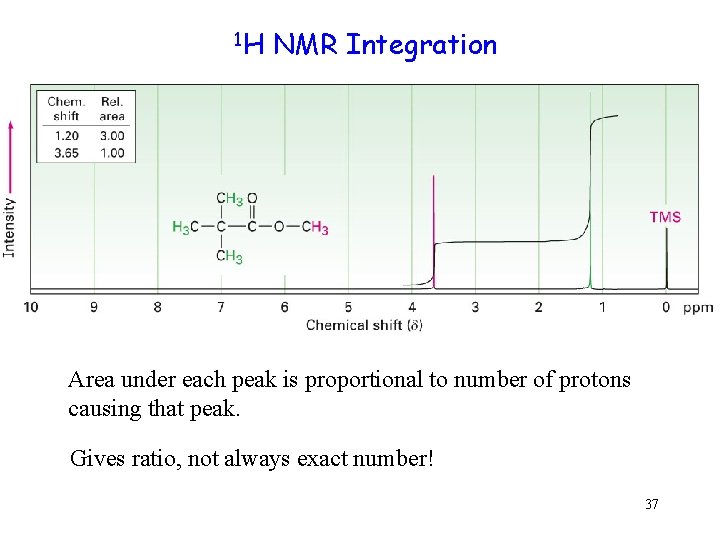
1 H NMR Integration Area under each peak is proportional to number of protons causing that peak. Gives ratio, not always exact number! 37

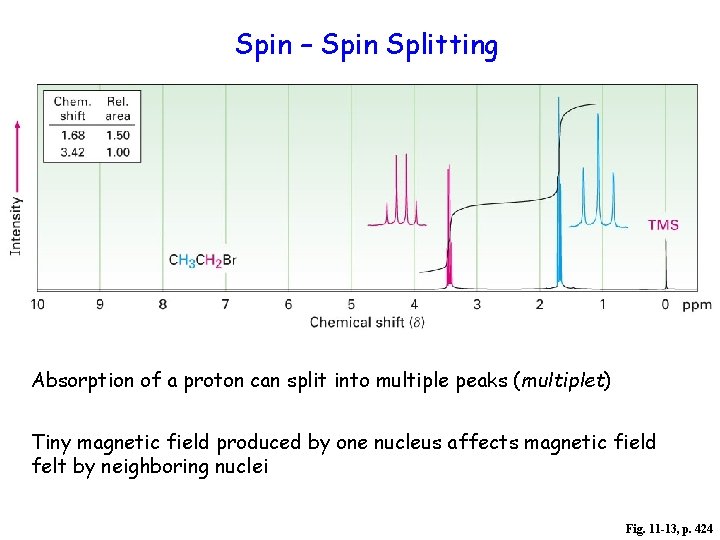
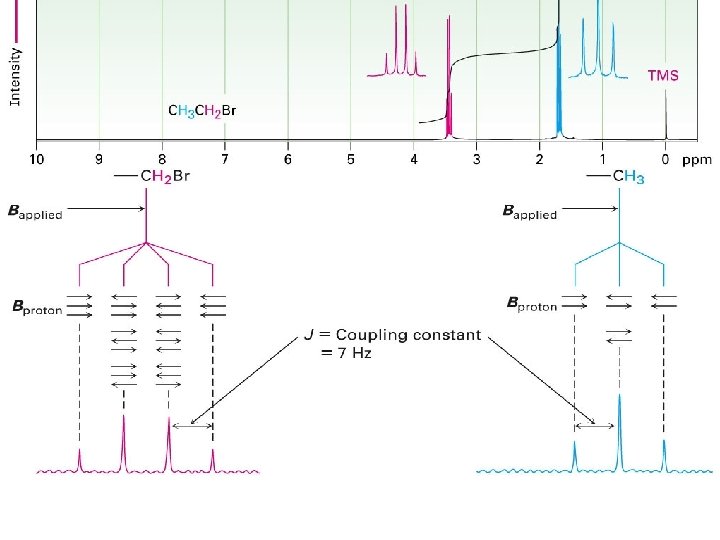
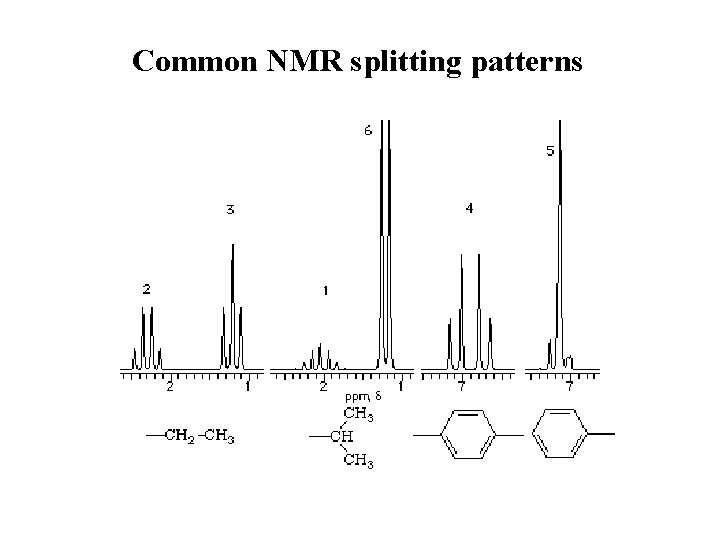
spin-spin splitting

Spin – Spin Splitting Absorption of a proton can split into multiple peaks (multiplet) Tiny magnetic field produced by one nucleus affects magnetic field felt by neighboring nuclei Fig. 11 -13, p. 424

Fig. 13 -13, p. 460

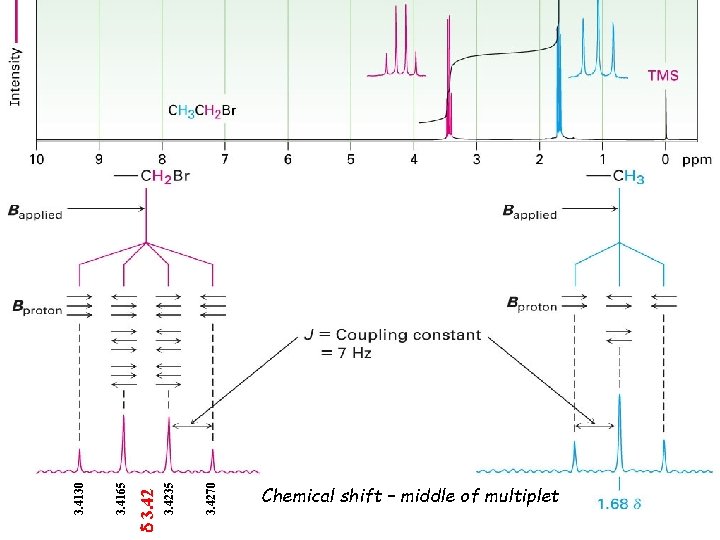
3. 4270 3. 4235 d 3. 42 3. 4165 3. 4130 Chemical shift – middle of multiplet Fig. 13 -13, p. 460

Common NMR splitting patterns

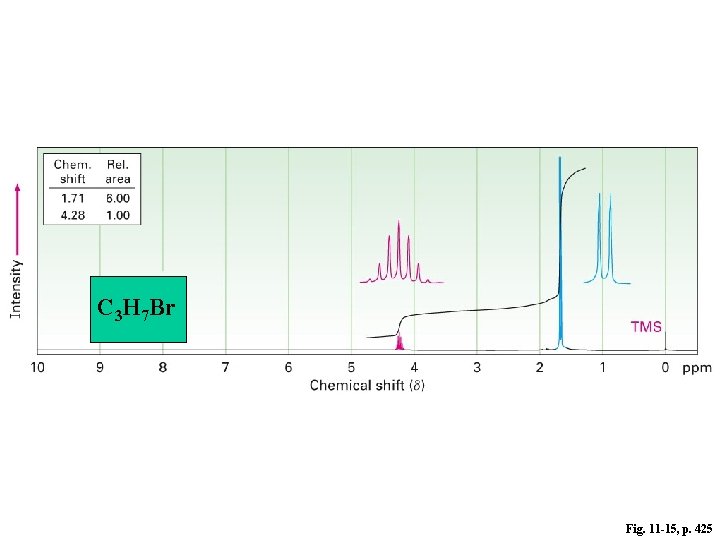
C 3 H 7 Br Fig. 11 -15, p. 425

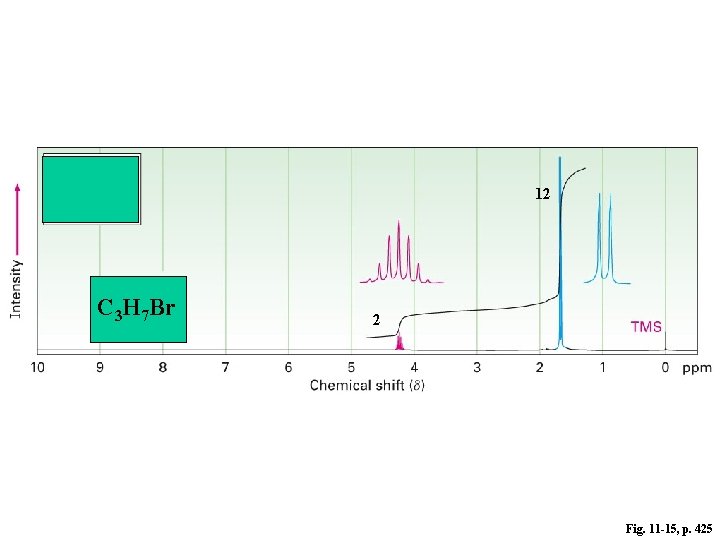
12 C 3 H 7 Br 2 Fig. 11 -15, p. 425

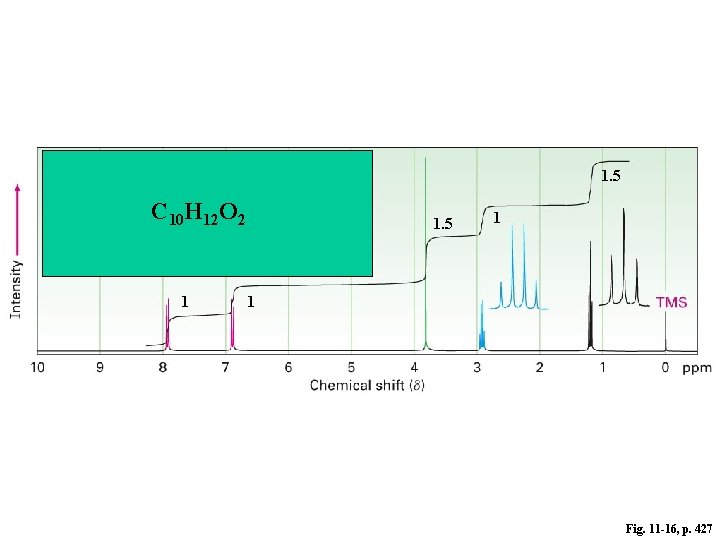
1. 5 C 10 H 12 O 2 1 1. 5 1 1 Fig. 11 -16, p. 427

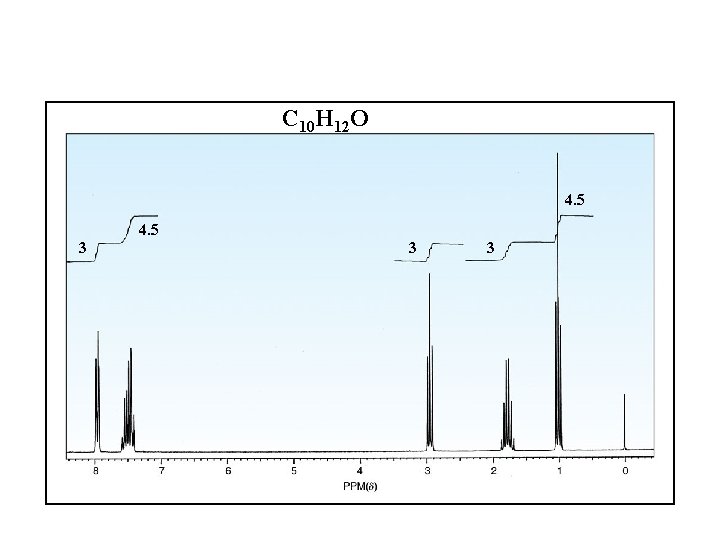
C 10 H 12 O 4. 5 3 3 3

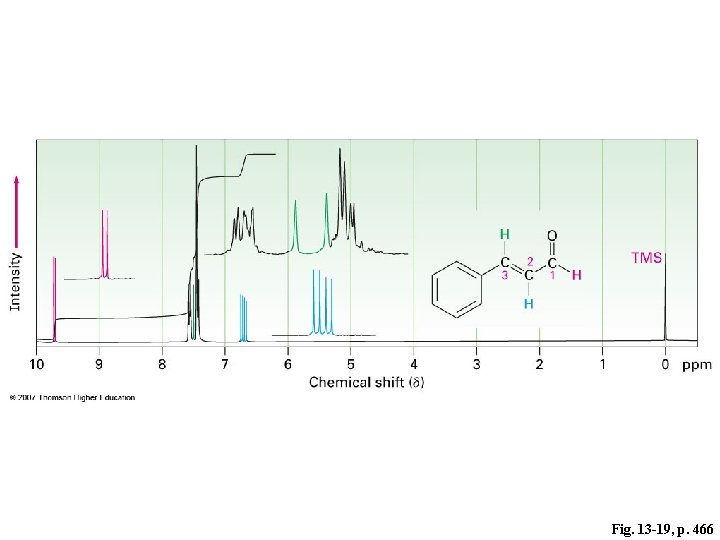
Fig. 13 -19, p. 466

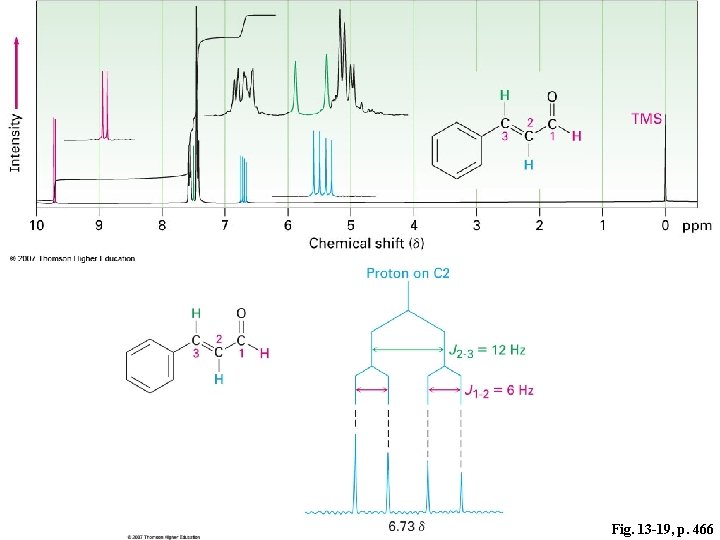
Fig. 13 -19, p. 466



p. 409