ISPCTN the IDe A States Pediatric Clinical Trials



















![Vaping rates [2017] in IDe. A states States Current Cigarettes (2017 - YRBS) Alaska Vaping rates [2017] in IDe. A states States Current Cigarettes (2017 - YRBS) Alaska](https://slidetodoc.com/presentation_image_h2/c2ca1c140c9687009bb4997a0315e535/image-29.jpg)



- Slides: 33

ISPCTN: the IDe. A States Pediatric Clinical Trials Network Dartmouth CO-OP PBRN January 26, 2020

ECHO (Environmental influences on Childhood Health Outcomes) - Mission & Vision • “To enhance the health of children for generations to come (through)…” –Becoming the pre-eminent child health research program –Observational and intervention research to inform highimpact programs, policies, and practices –Institute best practices for 21 st century team science


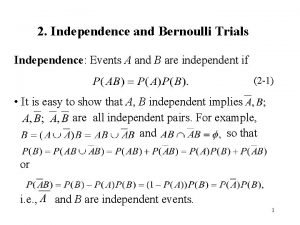
ISPCTN Organizational Structure NIH Director’s Office ECHO Program Office Matt Gilman ISPCTN Leadership Committee DCOC ISPCTN Steering Committee 17 ISPCTN Clinical Trials Units External Advisory Committee

ECHO Funding & Collaborators • Funding Agency: • Collaborators:

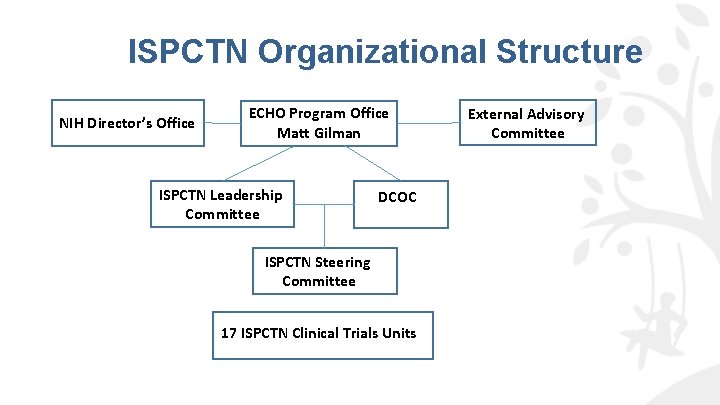
ECHO—A Nationwide Program ECHO Cohort Primary Site/Awardee ECHO Cohort Secondary Site ISPCTN Site/Awardee

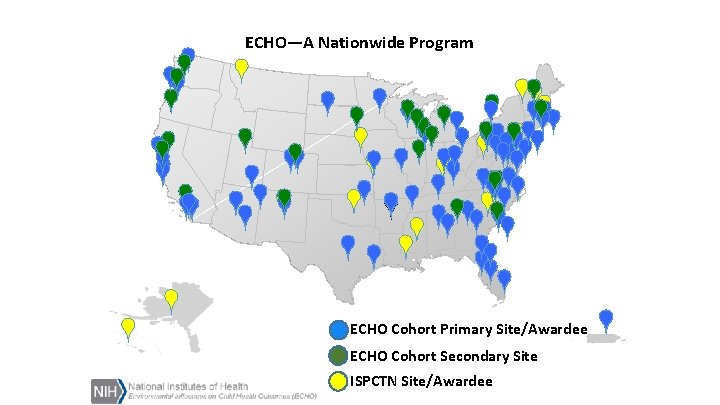
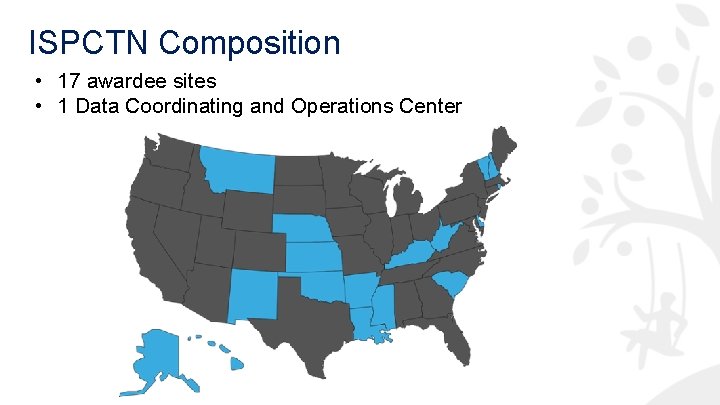
ISPCTN Composition • 17 awardee sites • 1 Data Coordinating and Operations Center

ISPCTN Mission & Vision • Provide medically underserved and rural populations with access to state-of-the-art clinical trials • Build national pediatric research capacity • Note: PBR and PBRNs are emphasized for ISPCTN v 2. 0

New Hampshire Site Team Paul Palumbo, MD, PI • ISPCTN Steering Committee • ISPCTN Leadership Committee Brian O’Sullivan, MD, Co-Investigator • Chair, ISPCTN VDORA clinical trial • Director, ISPCTN Manchester Carolyn Murray, MD , Co-Investigator • Director, ISPCTN Dartmouth COOP • Bonny Whalen, MD, Investigator ACT NOW investigator and consultant • Lou Guill, MD – Investigator VDORA lead for Dartmouth CTU • Komal Satti, MD – Investigator VDORA Investigator • • J. Dean Jarvis – Site/research coordinator ISPCTN Research Coordinators Committee CF Indoor Air Quality protocol investigator • • Mary Mc. Nally – Site/research coordinator ISPCTN Research Coordinators Committee CF Indoor Air Quality protocol investigator

ISPCTN Clinical Trials & Collaborations Pediatric Trials Network • POPS ** Original ISPCTN Studies • ACT NOW (with the Neonatal Research Network) ** • VDORA 1 ** • i. Am. Healthy • Indoor Air Quality for Children with CF (IAQ CF) ** • Barriers and Facilitators to ISPCTN Clinical Trials Participation

POPS • Pharmacokinetics of Understudied Drugs Administered to Children per Standard of Care (40 sites overall) • PTN (Pediatric Trials Network): –NICHD funded network to determine pediatric dosing • Capacity building for ISPCTN sites –All sites participating – 17 sites –POPS leadership includes 4 ISPCTN sites • ISPCTN sites matching/exceeding other POPS sites

Advancing Clinical Trials in Neonatal Opiate Withdrawal Syndrome (ACT NOW) Current Experience • Collaboration of ISPCTN & NICHD Neonatal Research Network

ACT NOW - CE • Aims: –Investigate variations in exposure, presentation, treatment and short-term outcomes for NOWS infants –Inform clinical trials • Methods: –Medical record review: July 1, 2016 –June 30, 2017 – 25 ISPCTN clinical sites from 17 awardees – 5 clinical sites from Ohio NRN sites – 1, 808 records • Data analysis in progress • Sub analyses: HCV prevalence and screening

ACT NOW: Clinical Trials in Development • Prospective Randomized Blinded Trial to Shorten Pharmacologic Treatment of Neonatal NOWS Adam Czynski, DO (RI) • Reducing Length of Time to Discharge for Infants with NOWS Using a Simplified Function-Based Assessment and Management Tool Leslie Young, MD (VT)

VDORA • New Hampshire –Brian O’Sullivan, MD –protocol concept and development, –network lead investigator

VDORA 1 • Vitamin D Supplementation in Children with Obesity Related Asthma • Phase 1: randomized, open-label, pharmacokinetic (PK) and safety study of oral vitamin D 3 • Phase 2: clinical trial of oral vitamin D 3 • Population: overweight/obese children with asthma • Enrollment (phase 1): –Started February 2019 –Completed – undergoing analysis for Phase 2 Dose

i. Am. Healthy • Feasibility Trial of m. Health Intervention –Aim: • Test validated group/family therapy m. Health intervention for rural ISPCTN overweight/obese children –Status: • NIH PRC approval • Sites identified • Enrollment opens next month • Full Intervention Trial anticipated in 2021 • Opportunity for PBRN

i. Am. Healthy • Dartmouth CO-OP application for Feasibility Trial –Criteria: –Minimum 40% Medicaid –Rural by RUCA codes –Have seen at least 100 children (6 -11), obese or overweight in past year OR >303 children total, with estimate of 33% obesity prevalence –Use EMR/ Cede to Central IRB • Canvassed COOP sites –Upper Valley Peds/ R. Yukica – 60% Medicaid/ Rural/ Met patient volume and other criteria • Outcome/ Lessons Learned

ISPCTN Pilot Study Protocol Development • Improving Indoor Air Quality for Children with Cystic Fibrosis –Feasibility Trial of HEPA Filtration intervention to Improve Indoor Air Quality (IAQ) for Children with Cystic Fibrosis • Vermont: § Kelly Cowan, MD: protocol concept and co-chair of protocol development writing group • New Hampshire: § Carolyn Murray, MD: protocol writing group § Laura Paulin, MD: protocol writing group • Considering external funding options

Other trials supported by our ISPCTN Pedi Trials Dept. • Neonatology –ICAF (Tyler Hartman) –RHO (Tyler Hartman) –MERCK (Tyler Hartman) –Brainstem functioning/OMT (Tyler Hartman) –NEARs 4 NEOS/CMAC (Neetu Singh) –Aerofact (Neetu Singh) – new –NAS and EEG (Melissa Schwedhelm and UVM) - new • Pediatrics/ PICU –RESQU (Matt Braga) –NEARS 4 KIDs (Sholeen Nett) –Other Nutrition studies (Marcy Singleton) –Paradigm (Sholeen Nett and Marcy Singleton) - new –RSV Antibody/Antigen (Peter Wright) –VALEAR (James Saunders) –And other studies with residents that we support

RFA-OD-19 -026: Clinical Sites for the ECHO IDe. A States Pediatric Clinical Trials Network - 2 (UG 1 Clinical Trial Required) • Application Due Date: December 2, 2019 Submitted! • Cooperative Agreement: substantial Federal scientific or programmatic involvement • 15 -20 awards anticipated (total of $7, 000, for fiscal year 2020) • 5 year awards • Additional protocol-specific funds available

• Participating Organization(s) –National Institutes of Health (NIH) –Office of the Director • Components of Participating Organizations –National Institute of Allergy and Infectious Diseases (NIAID) Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) National Institute on Drug Abuse (NIDA) National Institute of General Medical Sciences (NIGMS) National Institute of Neurological Disorders and Stroke (NINDS) • Office of Behavioral and Social Sciences Research (OBSSR)

CAPACITY DEVELOPMENT • Professional skills development: The network will focus on capacity building of professional skills among Senior faculty, Junior Faculty, and Research Coordinators –Senior Faculty Development Leader –Junior faculty pilot proposals

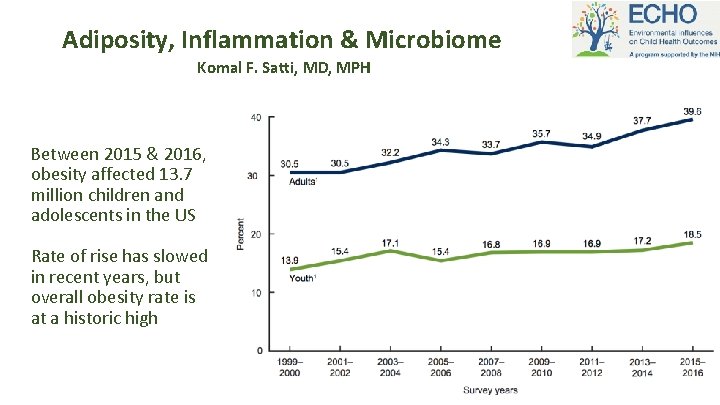
Adiposity, Inflammation & Microbiome Komal F. Satti, MD, MPH Between 2015 & 2016, obesity affected 13. 7 million children and adolescents in the US Rate of rise has slowed in recent years, but overall obesity rate is at a historic high

obesity Leptin, TNF alpha, IL-6, CRP, Adiponectin Inflammation Disease

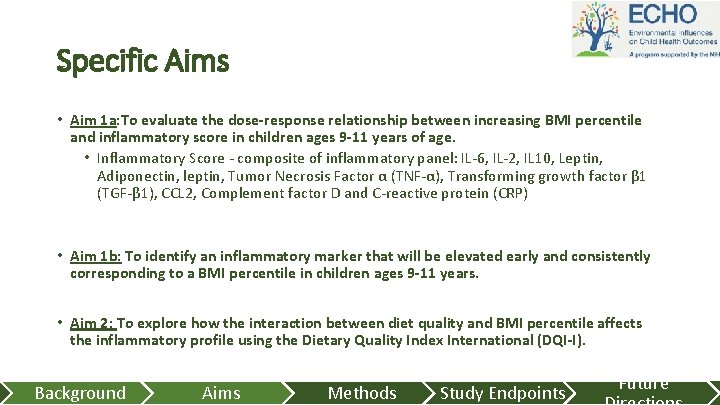
Specific Aims • Aim 1 a: To evaluate the dose-response relationship between increasing BMI percentile and inflammatory score in children ages 9 -11 years of age. • Inflammatory Score - composite of inflammatory panel: IL-6, IL-2, IL 10, Leptin, Adiponectin, leptin, Tumor Necrosis Factor α (TNF-α), Transforming growth factor β 1 (TGF-β 1), CCL 2, Complement factor D and C-reactive protein (CRP) • Aim 1 b: To identify an inflammatory marker that will be elevated early and consistently corresponding to a BMI percentile in children ages 9 -11 years. • Aim 2: To explore how the interaction between diet quality and BMI percentile affects the inflammatory profile using the Dietary Quality Index International (DQI-I). Background Aims Methods Study Endpoints Future

• Exploratory Aim: To assess the changing intestinal microbiome with increasing BMI percentile and inflammatory score. • Hypothesis: there is abundance of specific bacterial taxa in the intestinal microbiome with increasing BMI% in this age group. We would like to study how and when the bacterial diversity patterns change as children cross percentiles.

Clinical Trial Proposal: Comparative Interventions for Vaping Cessation Among Youth • Investigators: –Susanne Tanski (PI) and James Sargent – Koop Institute, Dartmouth –Laura Paulin (Pulmonary) and Zachary Goode (Cardiology) – both junior investigators at Dartmouth –Paul Palumbo, Dartmouth –Damon Vidrine, Ph. D and Jennifer Vidrine, Ph. D at the HL Moffitt Cancer Center, Tampa.
![Vaping rates 2017 in IDe A states States Current Cigarettes 2017 YRBS Alaska Vaping rates [2017] in IDe. A states States Current Cigarettes (2017 - YRBS) Alaska](https://slidetodoc.com/presentation_image_h2/c2ca1c140c9687009bb4997a0315e535/image-29.jpg)
Vaping rates [2017] in IDe. A states States Current Cigarettes (2017 - YRBS) Alaska Current Vaping – 2017 YRBS Frequent Vaping 15. 7 2. 7 Arkansas 13. 7 13. 9 2. 3 Delaware 6. 2 13. 6 2. 2 Hawaii 8. 1 25. 5 5. 1 Kansas 7. 2 10. 6 2. 4 Kentucky 14. 3 14. 1 2. 7 Louisiana 12. 3 12. 2 1. 7 Mississippi 7. 2 Missouri 9. 2 10. 9 2. 7 22. 5 3. 7 Montana Nebraska 7. 4 9. 4 1. 7 New Hampshire 7. 8 23. 8 5. 7 New Mexico 10. 6 24. 7 3. 7 Rhode Island 6. 1 20. 1 3. 7 South Carolina 10. 0 11. 9 2. 5 Vermont West Virginia 9. 3 12. 0 2. 6 14. 4 14. 3 3. 1 OVERALL US - 2017 8. 8 13. 2 3. 3 2019 YTS US 5. 8 27. 5 9. 4 Note that national vaping rates have more than doubled from 2017 to 2019, however state-level data is not yet available.

Treatment Conditions • Active Comparator: Quitline Treatment (QT). Brief advice to quit vaping (selfhelp materials) and connection to the standard tobacco Quitline program. Each state provides a version of the Quit. Line. • Experimental Comparator: Quitline Treatment plus NRT (QT-NRT). Quitline + nicotine replacement therapy (patches) and NRT lozenge or gum. • Experimental Comparator: Tech-based Treatment (TT). Technology-delivered automated treatment - texts, graphical messages and video clips that are personalized based on keywords provided by the participant. • Experimental Comparator: Tech-based Treatment plus NRT (TT-NRT).

Protocol Schema Age: 14 -22 years School-based ? Practice-based?

CO-OP PBRN Opportunities • Descriptive Catalog of Network and Composition of Practices • i. Am. Healthy Youth Obesity Intervention • Vaping Cessation • HCV – treatment and eradication • Opportunities through SYNERGY partnership • Your thoughts and recommendations are encouraged!!

 Pediatric trials network
Pediatric trials network Pengorganisasian dan revisi pesan bisnis
Pengorganisasian dan revisi pesan bisnis Berisi tentang
Berisi tentang Difference between inspection and audit
Difference between inspection and audit Role of statistician in clinical trials
Role of statistician in clinical trials Ohsu clinical trials office
Ohsu clinical trials office Mpn clinical trials
Mpn clinical trials York clinical trials unit
York clinical trials unit Nida clinical trial network
Nida clinical trial network Professor claire harrison
Professor claire harrison Clinical trials quality by design
Clinical trials quality by design Mrc clinical trials unit
Mrc clinical trials unit Prs registration
Prs registration Prs registration
Prs registration Site initiation visit ppt
Site initiation visit ppt Dhl clinical trials
Dhl clinical trials Randomization in statistics
Randomization in statistics Clinical trials.gov login
Clinical trials.gov login Clinical trials
Clinical trials Clinical trials gov api
Clinical trials gov api Clinical hysteria salem witch trials
Clinical hysteria salem witch trials Phs human subjects and clinical trials information
Phs human subjects and clinical trials information Iwr ivr clinical
Iwr ivr clinical Pediatric clinical nurse specialist programs
Pediatric clinical nurse specialist programs Southern states
Southern states Tyranny
Tyranny What were the 11 free states
What were the 11 free states Overcoming trials and temptations
Overcoming trials and temptations Random control trials
Random control trials Repeated bernoulli trials
Repeated bernoulli trials Salem witch trials rebecca nurse
Salem witch trials rebecca nurse Korean bridging trials
Korean bridging trials Malta football trials
Malta football trials Future search trials
Future search trials