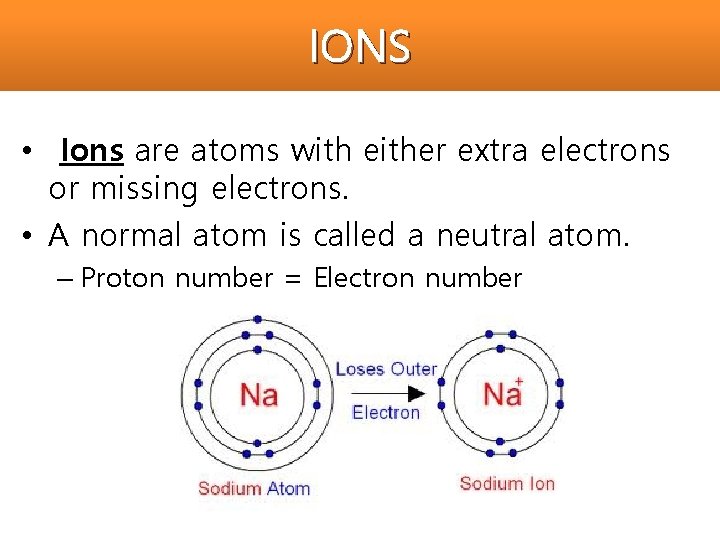
IONS Ions are atoms with either extra electrons
![Chlorine [Cl] +p= 17 -e= 17 -----0 charge Chlorine ion [Cl-] +p=17 -e=18 -------1 Chlorine [Cl] +p= 17 -e= 17 -----0 charge Chlorine ion [Cl-] +p=17 -e=18 -------1](https://slidetodoc.com/presentation_image_h2/2cb50985df28fdfbb7f3c0d6f9273b19/image-2.jpg)






- Slides: 18

IONS • Ions are atoms with either extra electrons or missing electrons. • A normal atom is called a neutral atom. – Proton number = Electron number
![Chlorine Cl p 17 e 17 0 charge Chlorine ion Cl p17 e18 1 Chlorine [Cl] +p= 17 -e= 17 -----0 charge Chlorine ion [Cl-] +p=17 -e=18 -------1](https://slidetodoc.com/presentation_image_h2/2cb50985df28fdfbb7f3c0d6f9273b19/image-2.jpg)
Chlorine [Cl] +p= 17 -e= 17 -----0 charge Chlorine ion [Cl-] +p=17 -e=18 -------1 charge

Find (a) The number of proton & electron. (b) Symbol 1. 2. 3. 4. Calcium ion Oxygen ion Magnesium ion Fluorine ion

Reacting chemicals

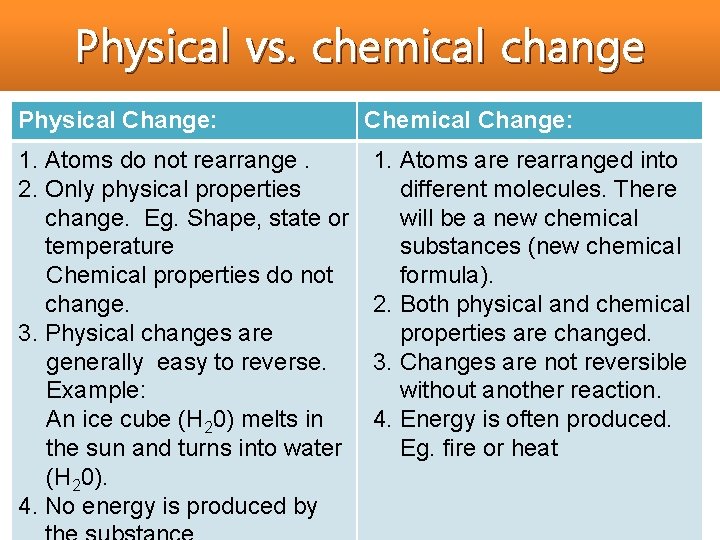
Physical vs. chemical change Physical Change: 1. Atoms do not rearrange. 2. Only physical properties change. Eg. Shape, state or temperature Chemical properties do not change. 3. Physical changes are generally easy to reverse. Example: An ice cube (H 20) melts in the sun and turns into water (H 20). 4. No energy is produced by Chemical Change: 1. Atoms are rearranged into different molecules. There will be a new chemical substances (new chemical formula). 2. Both physical and chemical properties are changed. 3. Changes are not reversible without another reaction. 4. Energy is often produced. Eg. fire or heat

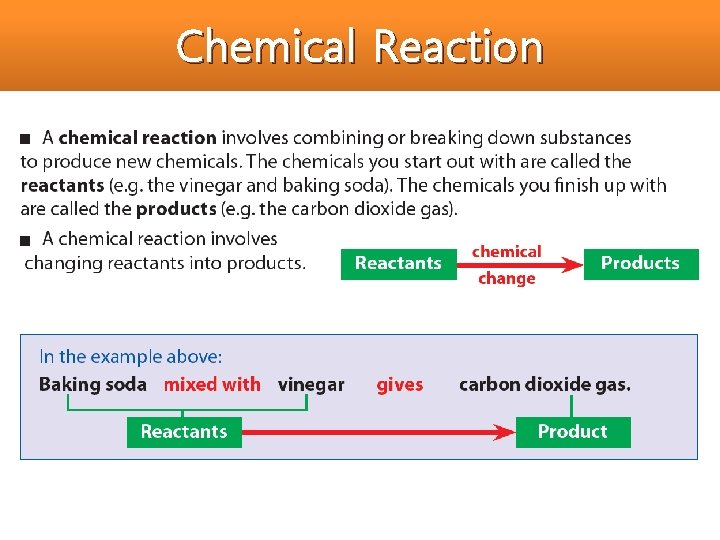
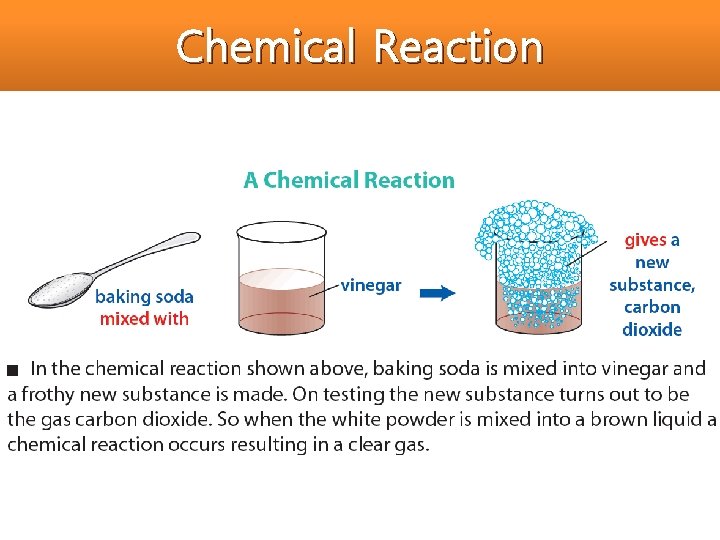
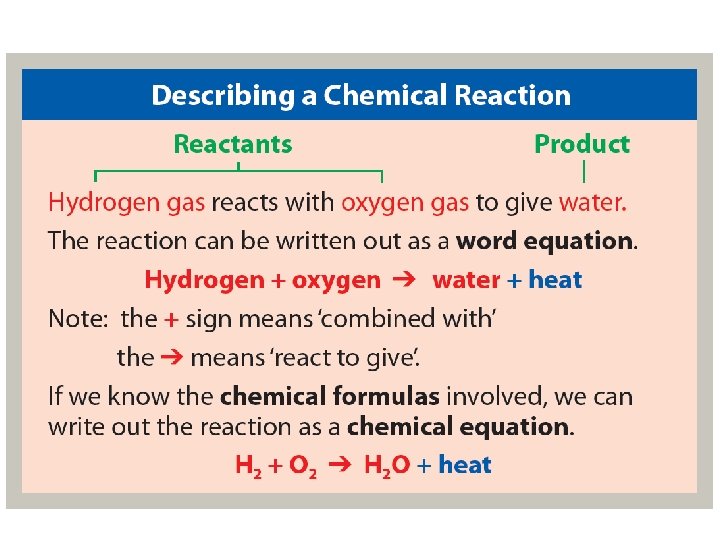
Chemical Reaction • All chemical reactions have two parts: 1. Reactants = the substances you start with 2. Products = the substances you end up with

Chemical Reaction

Chemical Reaction

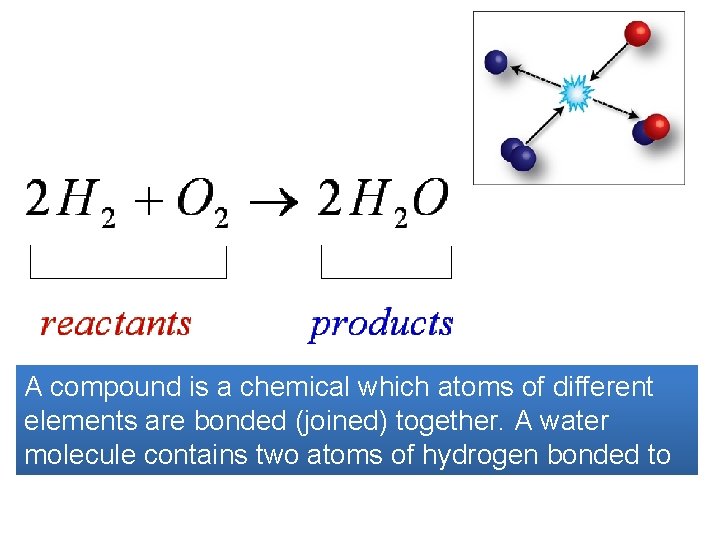
A compound is a chemical which atoms of different elements are bonded (joined) together. A water molecule contains two atoms of hydrogen bonded to each oxygen atom.


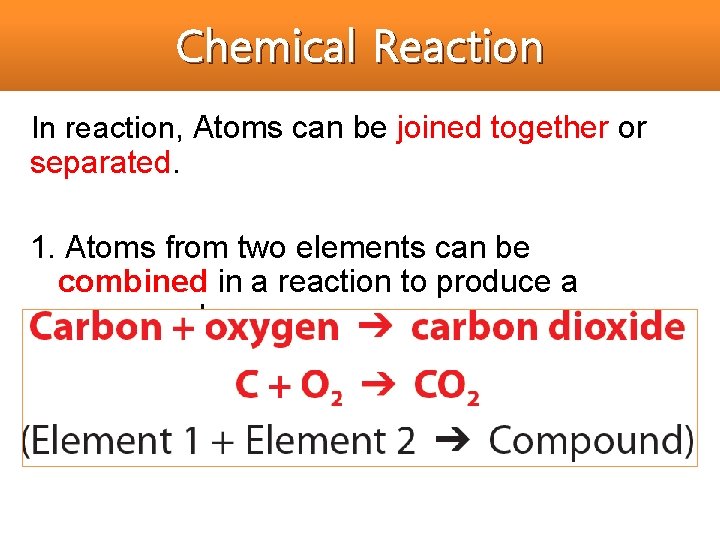
Chemical Reaction In reaction, Atoms can be joined together or separated. 1. Atoms from two elements can be combined in a reaction to produce a compound.

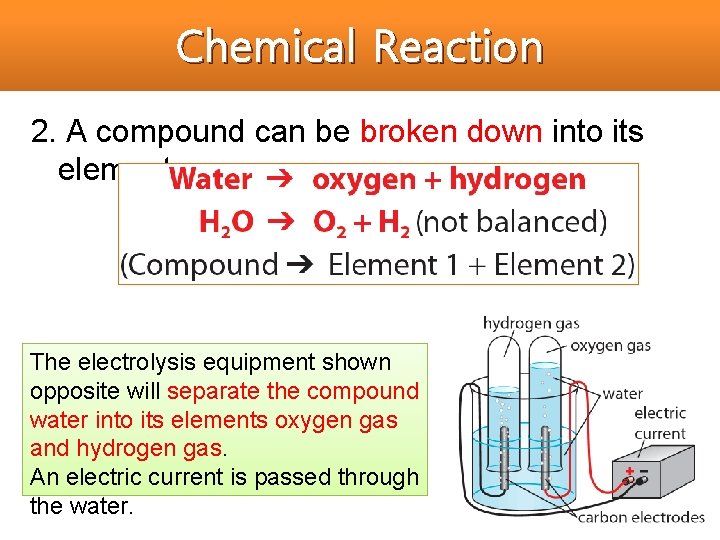
Chemical Reaction 2. A compound can be broken down into its elements. The electrolysis equipment shown opposite will separate the compound water into its elements oxygen gas and hydrogen gas. An electric current is passed through the water.

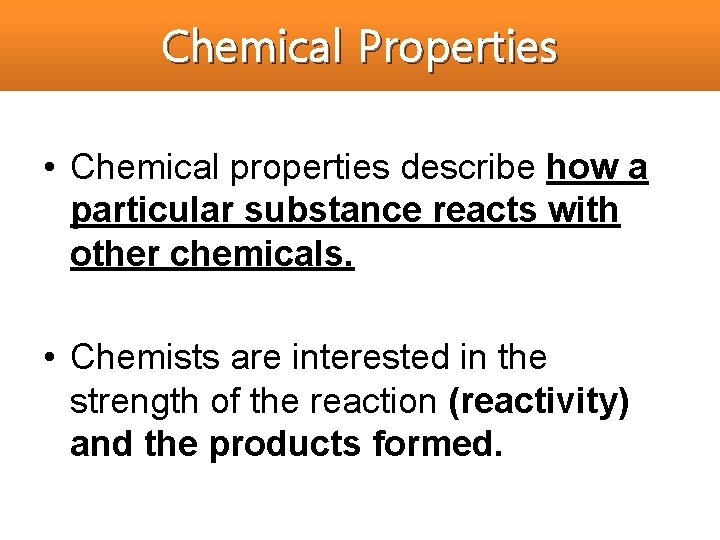
Chemical Properties • Chemical properties describe how a particular substance reacts with other chemicals. • Chemists are interested in the strength of the reaction (reactivity) and the products formed.

Key Chemical Properties

Acids and Bases • Acids are chemicals which … 1. Turn blue litmus paper red. 2. Taste sour (no not taste any chemicals in the lab). 3. Have hydrogen atom. • Bases are chemicals which … 1. Turn red litmus blue 2. Taste bitter & feel slippery or soapy 3. Have hydroxide (OH-) ions.

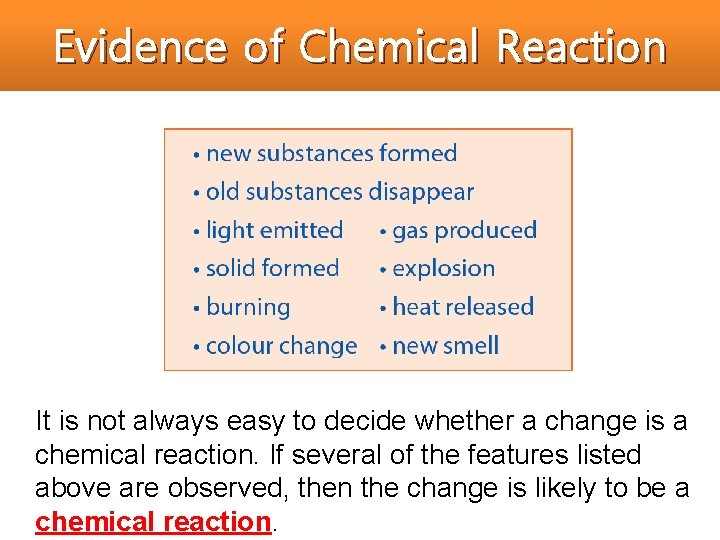
Evidence of Chemical Reaction It is not always easy to decide whether a change is a chemical reaction. If several of the features listed above are observed, then the change is likely to be a chemical reaction.

Combustion takes place when fuel, most commonly a fossil fuel, reacts with the oxygen in air to produce heat. Along with heat, CO 2 (carbon dioxide) and H 20 (water) are created as byproducts of the exothermic reaction.

Combustion Inside the space shuttle’s main engine hydrogen and oxygen gases react in a controlled explosion called combustion. The product is very hot steam. The rapidly expanding hot gas is forced out of the back of the rocket,