Ions in Aqueous Solutions and Colligative Properties Chapter


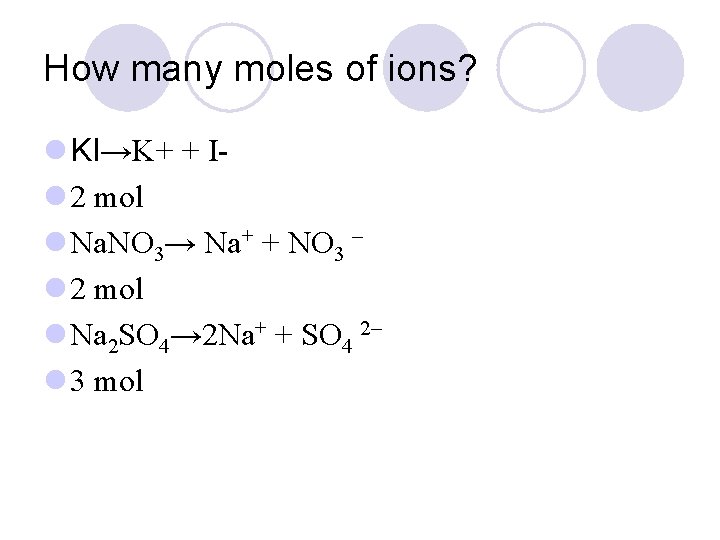








- Slides: 24

Ions in Aqueous Solutions and Colligative Properties Chapter 13 Chemistry

Compounds in Aqueous Solutions 13 -1

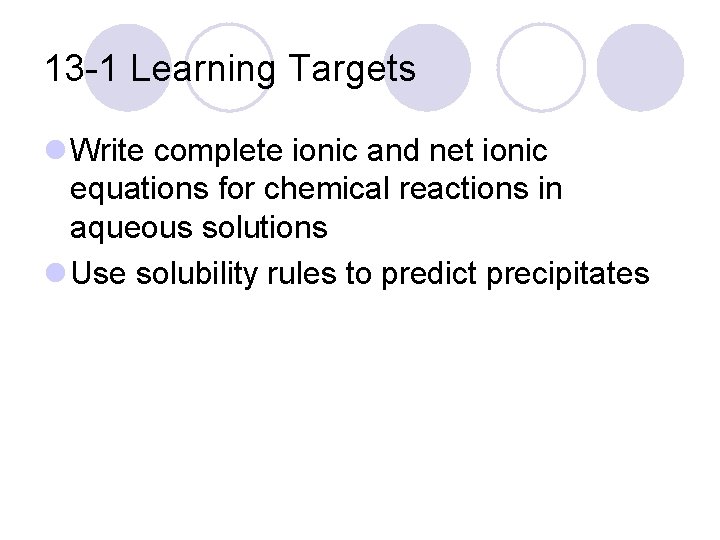
13 -1 Learning Targets l Write complete ionic and net ionic equations for chemical reactions in aqueous solutions l Use solubility rules to predict precipitates

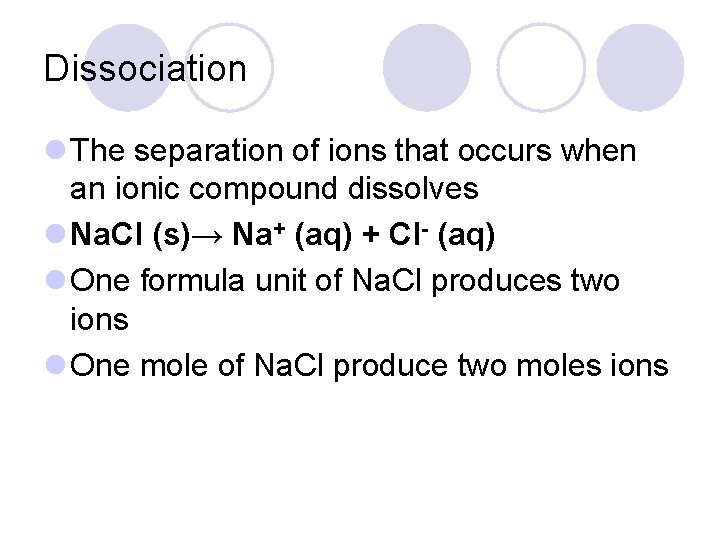
Dissociation l The separation of ions that occurs when an ionic compound dissolves l Na. Cl (s)→ Na+ (aq) + Cl- (aq) l One formula unit of Na. Cl produces two ions l One mole of Na. Cl produce two moles ions

l Write the equation for the dissolution of magnesium chlorate in water. How many moles of ions are produced for every 1 mol of magnesium chlorate dissolved? l Mg(Cl. O 3)2 (s)→ Mg 2+ (aq) + 2 Cl. O 3 - (aq) l 3 mol
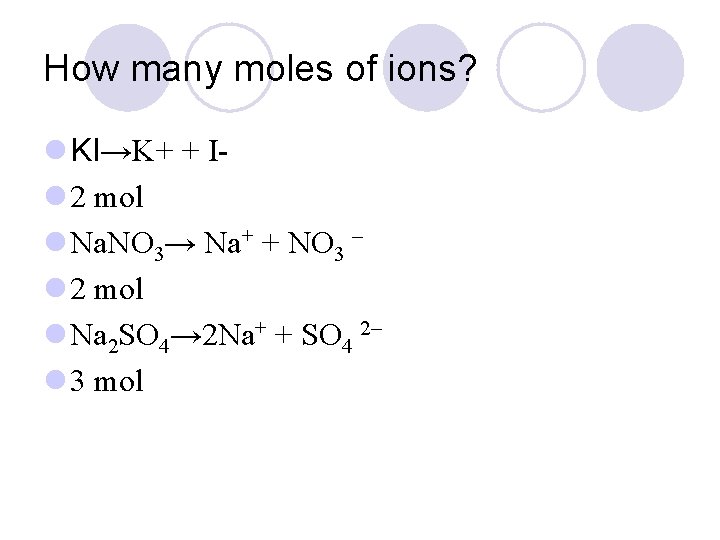
How many moles of ions? l KI→K+ + Il 2 mol l Na. NO 3→ Na+ + NO 3 – l 2 mol l Na 2 SO 4→ 2 Na+ + SO 4 2– l 3 mol

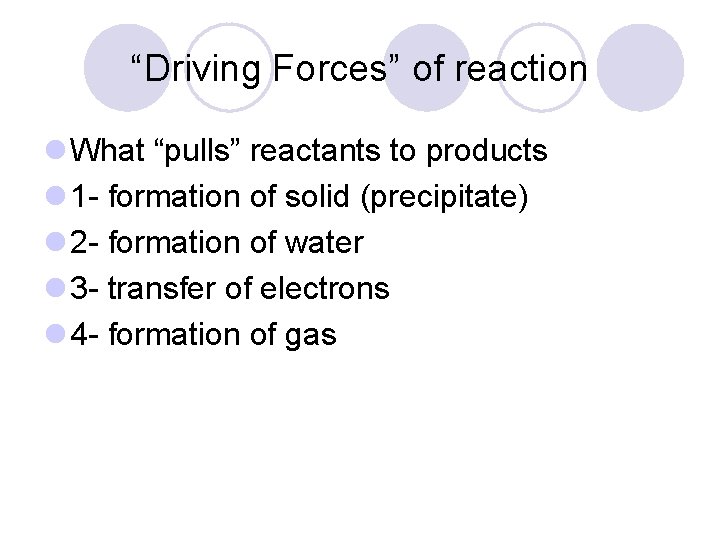
“Driving Forces” of reaction l What “pulls” reactants to products l 1 - formation of solid (precipitate) l 2 - formation of water l 3 - transfer of electrons l 4 - formation of gas


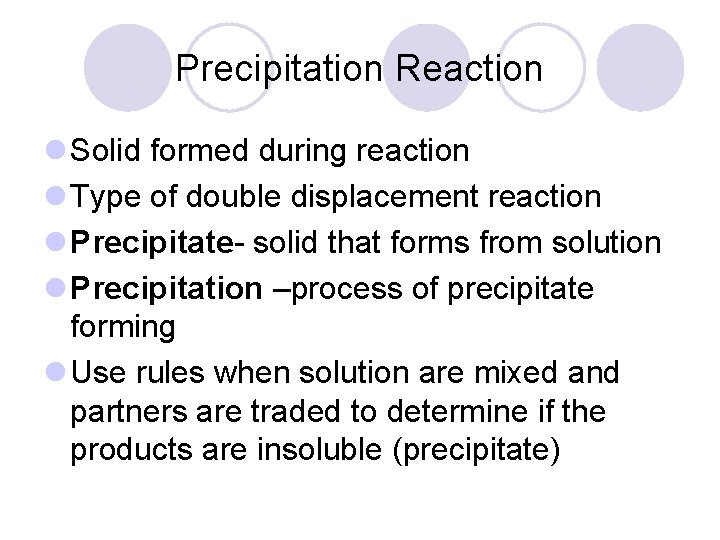
Precipitation Reaction l Solid formed during reaction l Type of double displacement reaction l Precipitate- solid that forms from solution l Precipitation –process of precipitate forming l Use rules when solution are mixed and partners are traded to determine if the products are insoluble (precipitate)

Solubility Rules l Soluble- dissolves in water l Slightly soluble- insoluble in water, only a few ions form l No compound is completely insoluble l Dissociation equation cannot be written for insoluble compounds

l Dissociation equation for soluble substance l Na 2 SO 4 (s)→ 2 Na+ (aq) + SO 42 - (aq)

l 7 - Most hydroxide compounds are only slightly soluble (insoluble). Exceptions: Na. OH and KOH

Are these soluble? l KCl l Soluble l Fe. S l Insoluble l Ag. Cl l Insoluble l Ca. S l soluble l Cd. S l Insoluble l NH 4 Br l Soluble l Na. SO 4 l soluble l Ba. SO 4 l insoluble

l Review ions and polyatomic ions l Will make writing equations easier and faster

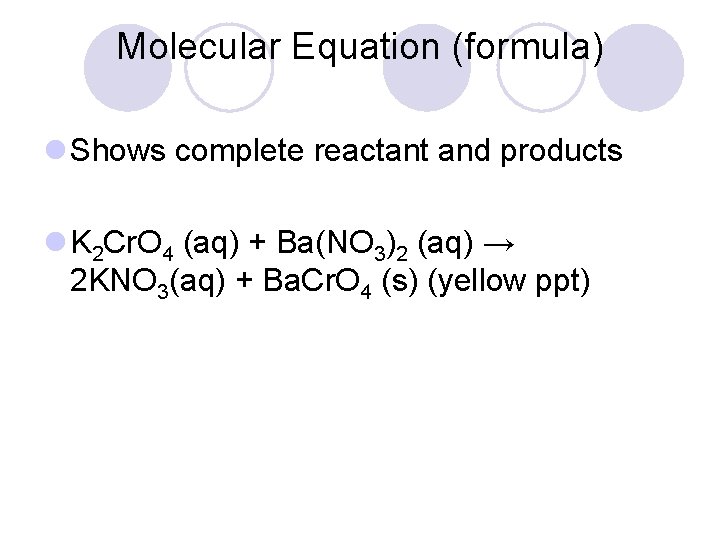
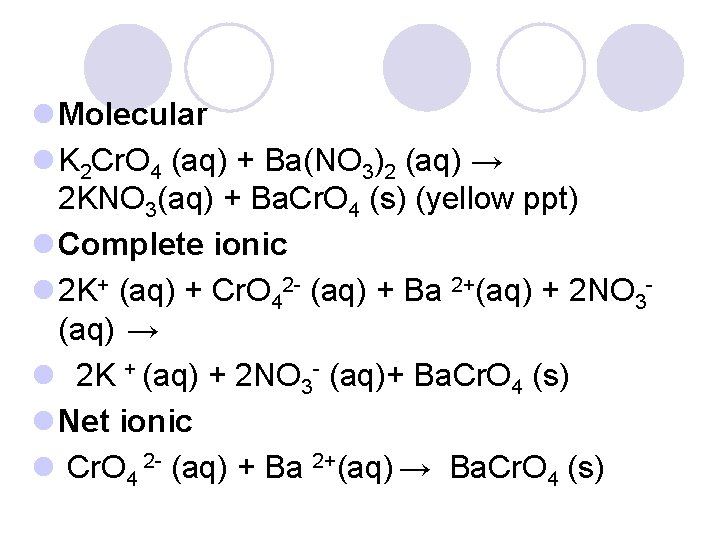
Molecular Equation (formula) l Shows complete reactant and products l K 2 Cr. O 4 (aq) + Ba(NO 3)2 (aq) → 2 KNO 3(aq) + Ba. Cr. O 4 (s) (yellow ppt)

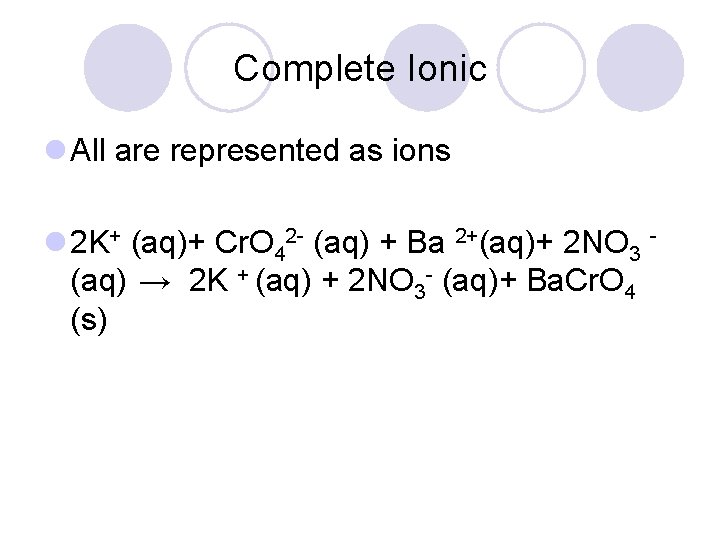
Complete Ionic l All are represented as ions l 2 K+ (aq)+ Cr. O 42 - (aq) + Ba 2+(aq)+ 2 NO 3 (aq) → 2 K + (aq) + 2 NO 3 - (aq)+ Ba. Cr. O 4 (s)

Net Ionic l Includes only those ions directly involved in the reaction (what will form the precipitate (ppt)) l No spectator ions present l Spectator ions- do not take part in chemical rxn ¡Found in solution before and after rxn l Cr. O 4 2 - (aq) + Ba 2+(aq) → Ba. Cr. O 4 (s)

l Molecular l K 2 Cr. O 4 (aq) + Ba(NO 3)2 (aq) → 2 KNO 3(aq) + Ba. Cr. O 4 (s) (yellow ppt) l Complete ionic l 2 K+ (aq) + Cr. O 42 - (aq) + Ba 2+(aq) + 2 NO 3(aq) → l 2 K + (aq) + 2 NO 3 - (aq)+ Ba. Cr. O 4 (s) l Net ionic l Cr. O 4 2 - (aq) + Ba 2+(aq) → Ba. Cr. O 4 (s)

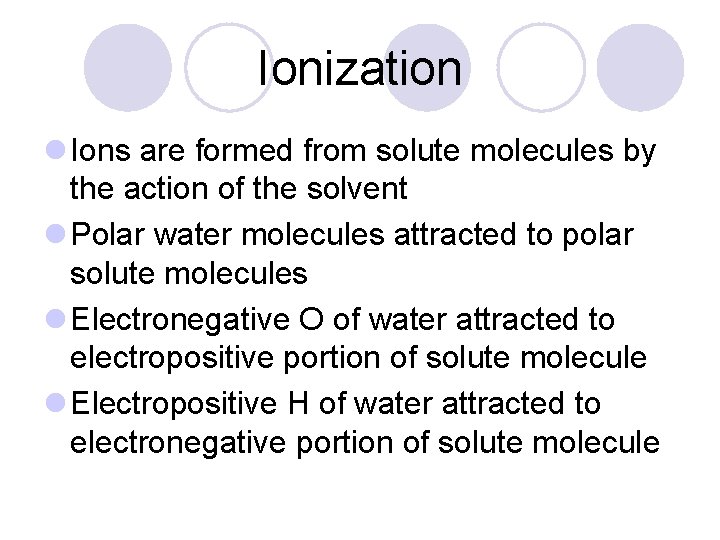
Ionization l Ions are formed from solute molecules by the action of the solvent l Polar water molecules attracted to polar solute molecules l Electronegative O of water attracted to electropositive portion of solute molecule l Electropositive H of water attracted to electronegative portion of solute molecule

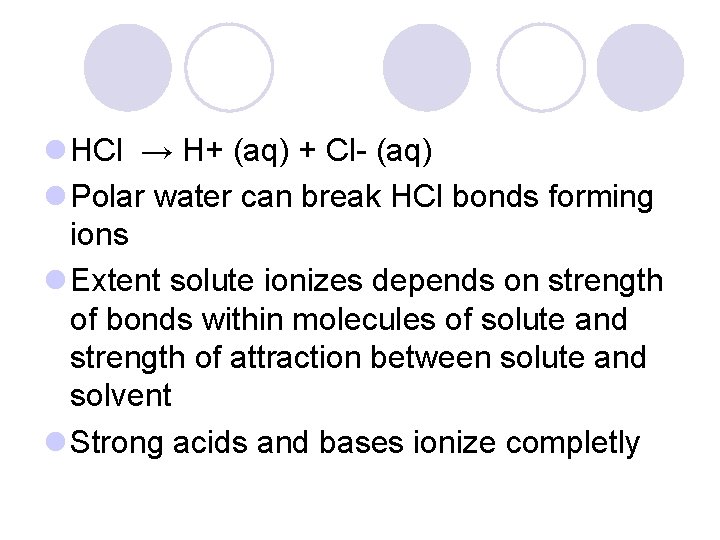
l HCl → H+ (aq) + Cl- (aq) l Polar water can break HCl bonds forming ions l Extent solute ionizes depends on strength of bonds within molecules of solute and strength of attraction between solute and solvent l Strong acids and bases ionize completly

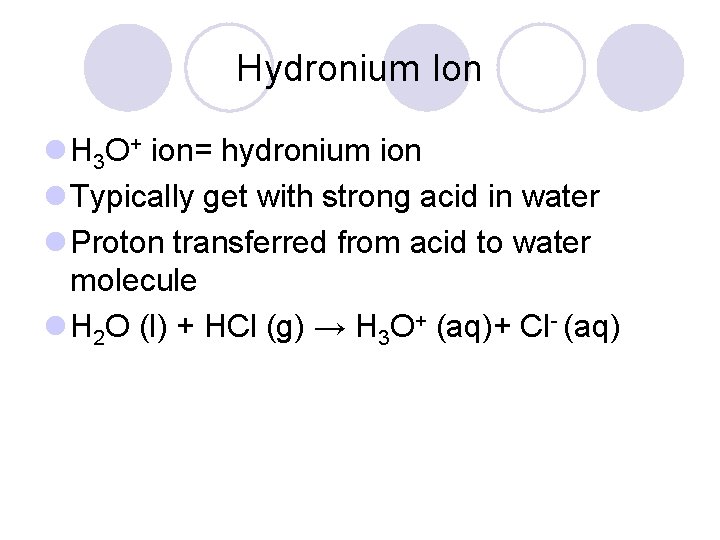
Hydronium Ion l H 3 O+ ion= hydronium ion l Typically get with strong acid in water l Proton transferred from acid to water molecule l H 2 O (l) + HCl (g) → H 3 O+ (aq)+ Cl- (aq)

Electrolyte l Provides ions in water solution = a better conductor l Strong electrolyte- Any compound of which all or almost all dissolved compound exists as ions in aq solution l All ionic compounds l Hydrogen halide (HCl, HBr, HI)

l Weak Electrolytes l A compound of which a relatively small amount of the dissolved compound exists as ion in aq solution ¡HF, organic acids

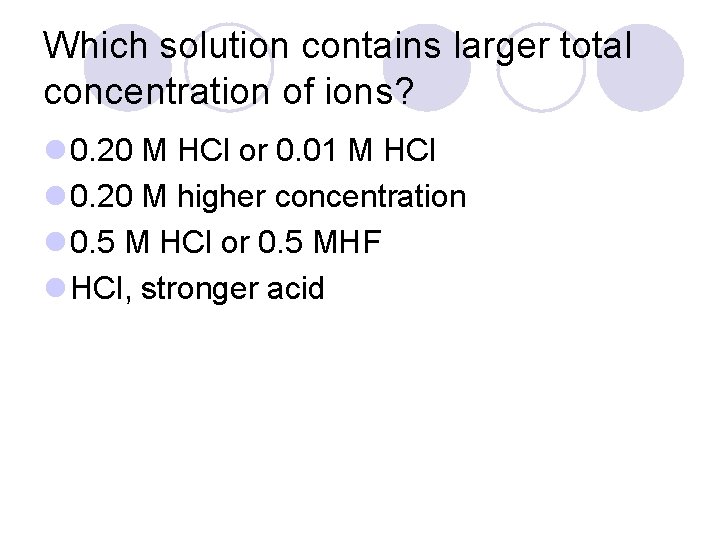
Which solution contains larger total concentration of ions? l 0. 20 M HCl or 0. 01 M HCl l 0. 20 M higher concentration l 0. 5 M HCl or 0. 5 MHF l HCl, stronger acid