Chapter 14 Section 14 4 Colligative Properties of

















- Slides: 22

Chapter 14 Section 14. 4 Colligative Properties of Solutions

Electrolytes and Colligative Properties Colligative properties are physical properties of solutions that are affected by the number of particles but not by the identity of dissolved solute particles. Colligative means depending on the collection Colligative properties depend on the number of solute particles in a solution. Colligative properties include: 1 - vapor pressure lowering 2 - boiling point elevation 3 - freezing point depression 4 - osmotic pressure.

n n Electrolytes: compounds that are dissociate in water to form ions in the solution and conduct electricity. Ionic compounds(like Na. Cl, KCl, Ca. Cl 2) are electrolytes because they dissociate in water to form a solution that include ions conducts electricity. Electrolytes that produce many ions in solution are strong electrolytes. Some molecular compounds are also electrolytes.

Non electrolytes: compounds that do not dissociate in water to form ions in the solution and do not conduct electricity. Many molecular compounds do not ionize when dissolved, and do not conduct electricity like C 6 H 12 O 6 1 - Vapor Pressure Lowering: is due to the number of solute particles in solution and is a colligative property of solutions. n vapor pressure is the pressure exerted in a closed container by liquid particles that have escaped the liquid’s surface and entered the gaseous state. n The greater the number of solute particles, the lower the vapor pressure. n Q: Which will has more effect on the vapor pressure , strong or weak electrolytes compounds? Why? n Strong ones, as they will produce more dissociated ions and increase number of particles in the solution

n n n Adding a nonvolatile solute to a solvent lowers the solvent’s vapor pressure. Nonvolatile solute: is the one that has little tendency to become a gas Volatile solute: is the one that has high tendency to become a gas

n n 2 - Boiling Point Elevation: When a nonvolatile solute lowers the vapor pressure of a solvent, the boiling point is also affected. The boiling will not occur unless the vapor pressure equals the atmospheric pressure More heat is needed to supply additional kinetic energy to raise number of solvent particles escaping the solvent surface and as a result increasing the vapor pressure to equalize atmospheric pressure.

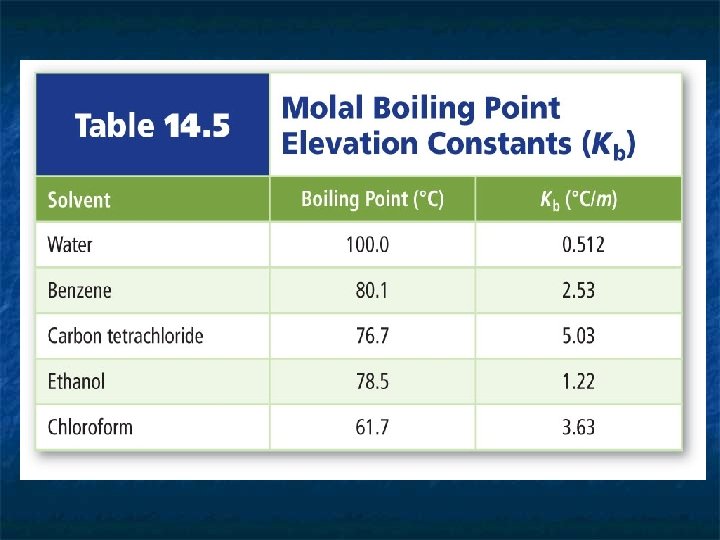
The temperature difference between a solution’s boiling point and a pure solvent's boiling point is called the boiling point elevation n The greater the number of solute particles, the higher the boiling point. n ΔTb = Kbm where ΔTb is the boiling point elevation, Kb is the molal boiling point elevation constant, and m represents molality. n The molal boiling point elevation constant Kb Is the difference in boiling points between 1 m nonvolatile, non electrolytes solution and pure solvent n


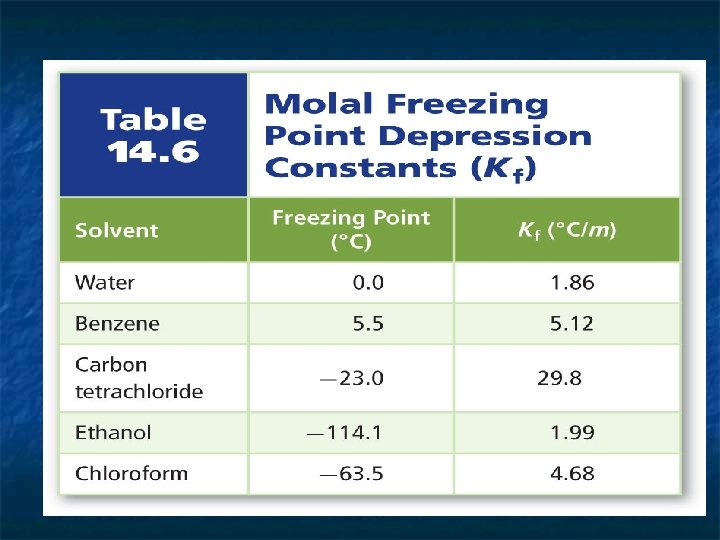
n n 3 - Freezing Point Depression: At a solvent's freezing point temperature, particles no longer have sufficient kinetic energy to overcome interparticle attractive forces. The freezing point of a solution is always lower than that of the pure solvent. Solute particles interfere with the attractive forces among solvent particles. This prevents the solvent from entering the solid state at its normal freezing point


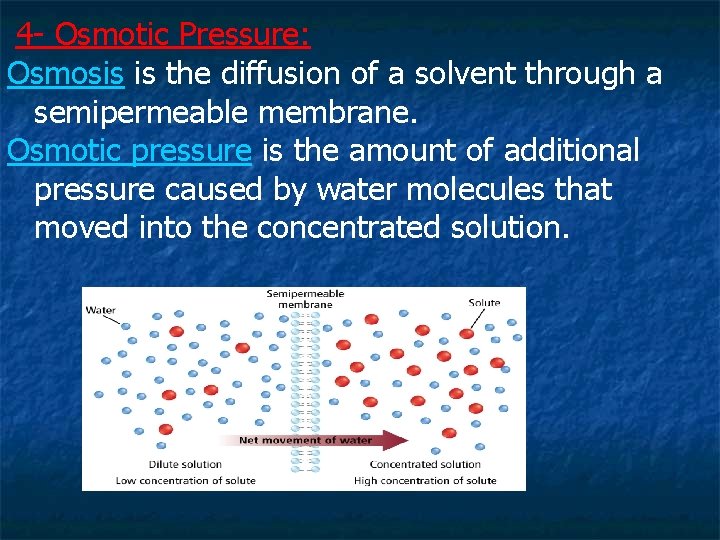
4 - Osmotic Pressure: Osmosis is the diffusion of a solvent through a semipermeable membrane. Osmotic pressure is the amount of additional pressure caused by water molecules that moved into the concentrated solution.


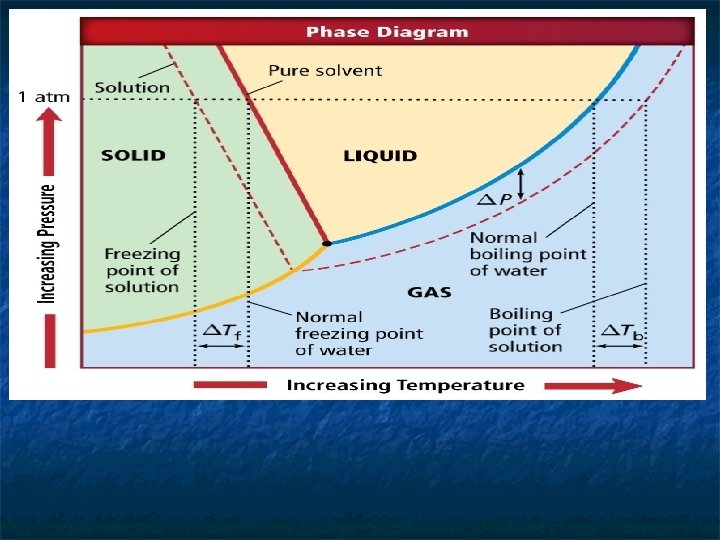
n n n n 1. What variables are plotted on the phase diagram? pressure and temperature 2. What solvent is represented in the phase diagram? water 3. What phases of the solvent are represented in the diagram? gas, liquid, and solid 4. What do the solid lines represent? The solid lines represent the pressures and temperatures at which two phases of the pure solvent (water) coexist. 5. What is the term applied to a solution in which water is the solvent? aqueous solution 6. What do the dashed lines represent? The dashed lines represent the pressures and temperatures at which two phases of the aqueous solution coexist 7. At each temperature, what does ΔP represent? ΔP represents the lowering of the vapor pressure due to the addition of the solute.

8. At any temperature, how does the vapor pressure of the aqueous solution compare with the vapor pressure of the pure solvent? The vapor pressure of the aqueous solution is always lower than the vapor pressure of the pure solvent. 9. Will a solution boil at the same temperature as the pure solvent under normal atmospheric pressure? Explain. No; a liquid boils when its vapor pressure equals atmospheric pressure. Because the vapor pressure of a solution is always less than the vapor pressure of the pure solvent at the same temperature, the solution can never boil under normal atmospheric pressure at the same temperature as the pure solvent. n n n 10. What must you do to the temperature of a solution to make it boil if it is at the boiling point of the pure solvent under normal atmospheric pressure? raise its temperature 11. How does the freezing point of a solution compare with the freezing point of the pure solvent at the same pressure? The freezing point of the solution is lower.

State change Result from addition of a solute Vapor Pressure The addition of a solute lowers the VP Boiling Point The addition of a solute raises the BP Freezing Point The addition of a solute lowers the FP Osmotic Pressure The addition of a solute raised the OP

n n Explain why a solution has a higher boiling point than that of the pure solvent? Solute particles in solution decrease the vapor pressure above the solution. Because a solution boils when its vapor pressure equals the external pressure, this decrease in vapor results in the need for a higher temperature in order for the solution to boil

Nonvolatile solutes ____ the vapor pressure of a solution. A. increase B. decrease C. do not change D. unpredictably change

Colligative properties of a solution depend on: A. the type of solute B. the type of solvent C. the vapor pressure of the solvent D. the number of particles of solute

The addition of a nonvolatile solute to a solution: A. increases the freezing point of the solution B. increases the vapor pressure of the solution C. lowers the boiling point of the solution D. decreases vapor pressure of the solution

Which is NOT a colligative property? A. heat of solution B. boiling point elevation C. vapor pressure lowering D. freezing point depression

Nonvolatile solutes _____ the boiling point of a solution. A. increase B. decrease C. do not change D. unpredictably change

End of section 14. 4