Colligative Properties of Solutions Colligative comes from the












- Slides: 23

Colligative Properties of Solutions Colligative comes from the Latin colligatus meaning bound together.

Solutions vs. compounds • Recall that compounds are substances consisting of two or more different chemical elements combined in fixed and definite proportions and held together by chemical bonds. Compounds can only be separated into simpler substances by undergoing chemical reactions during which chemical bonds are broken and/or formed. • Also recall that solutions are homogeneous mixtures of two or more substances. Because each substance in a solution retains its identity and the mixture can be separated by physical means, no chemical bonds have been broken or formed so therefore no new compounds were formed.

Solutions vs. compounds • Also remember that compounds have different properties (e. g. , boiling point, freezing point, electrical conductivity) than the properties of the individual elements of which they are composed. Water has very different properties than do oxygen gas or hydrogen gas. • Here’s a similarity between compounds and solutions: Solutions also have different properties than either the solute(s) or the solvent used to make the solution. Salt water has different properties from either solid sodium chloride or pure liquid water.

Physical and Chemical Properties • Examples of physical properties (no change in chemical identity of a substance) are color, volume, mass, volume, density, boiling point, and freezing point. • Examples of chemical properties (must undergo a chemical reaction to observe) are flammability, reactivity, heat of combustion, etc.

Physical Properties • Physical properties were subdivided into INTENSIVE (doesn’t depend on the amount, such as density and concentration) and EXTENSIVE (depends on the amount, such as mass and volume). • For solutions, intensive properties are further subdivided into COLLIGATIVE and NON-COLLIGATIVE.

Properties of Solutions • Colligative properties depend only on the number of dissolved particles of solute in solution and not on their identity. Noncolligative properties depend only on the identities of the dissolved species and the solvent. • For colligative properties, the ratio of the number of solute particles to the number of solvent molecules in a solution can be related to various units for concentration of solutions such as molarity and molality.

Colligative vs. Non-colligative • For instance, compare the properties of a 1. 0 M aqueous sugar solution to a 0. 5 M solution of table salt (Na. Cl) in water. • Even though the concentration of sodium chloride is half that of the sucrose concentration, both solutions have exactly the same number of dissolved particles because each sodium chloride unit creates two particles when it dissolves--a sodium ion, Na+, and a chloride ion, Cl-.

Colligative vs. Non-colligative • Since they have the same number of dissolved particles, any difference in the properties of the sugar and salt solutions must be due to a non-colligative property. • Both solutions have the same freezing point, boiling point, vapor pressure, and osmotic pressure because those colligative properties of a solution only depend on the number of dissolved particles.

Colligative vs. Non-colligative • What properties are different? Taste is one! The sugar solution is sweet and the salt solution tastes salty. • Another non-colligative property is the color of a solution. A 0. 5 M solution of Cu. SO 4 is bright blue in contrast to the colorless salt and sugar solutions. • Other non-colligative properties include viscosity, surface tension, and solubility.

Colligative Properties Colligative properties depend upon the concentration of solutions: 1. Vapor pressure (the pressure of a vapor in thermodynamic equilibrium with its condensed phases in a closed container) 2. Boiling point 3. Freezing point 4. Osmotic pressure (the tendency of a pure solvent to move into a solution through a semi-permeable, one-way membrane that doesn’t allow the solution to flow backwards into the pure solvent)

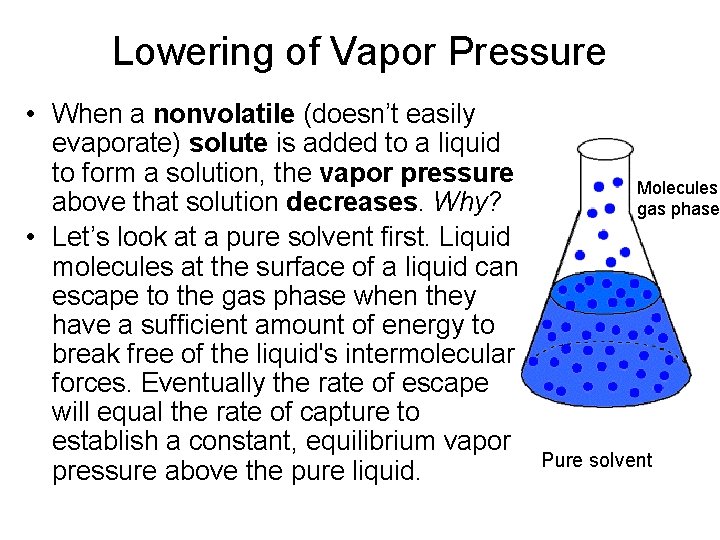
Lowering of Vapor Pressure • When a nonvolatile (doesn’t easily evaporate) solute is added to a liquid to form a solution, the vapor pressure above that solution decreases. Why? • Let’s look at a pure solvent first. Liquid molecules at the surface of a liquid can escape to the gas phase when they have a sufficient amount of energy to break free of the liquid's intermolecular forces. Eventually the rate of escape will equal the rate of capture to establish a constant, equilibrium vapor pressure above the pure liquid. Molecules gas phase Pure solvent

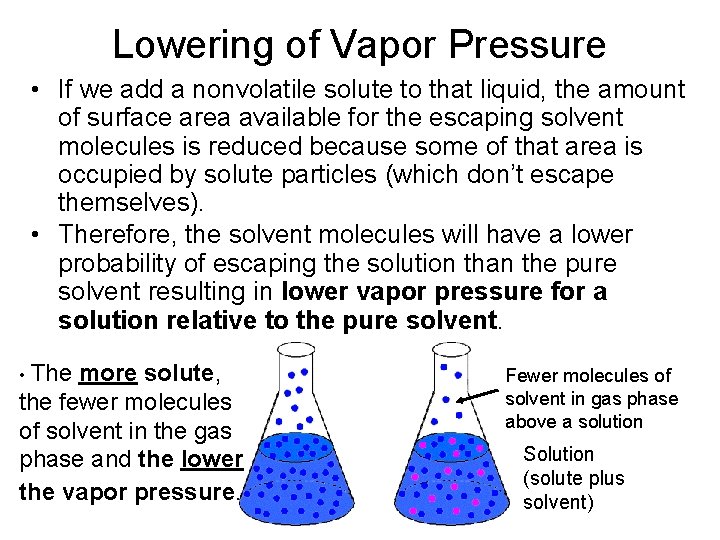
Lowering of Vapor Pressure • If we add a nonvolatile solute to that liquid, the amount of surface area available for the escaping solvent molecules is reduced because some of that area is occupied by solute particles (which don’t escape themselves). • Therefore, the solvent molecules will have a lower probability of escaping the solution than the pure solvent resulting in lower vapor pressure for a solution relative to the pure solvent. • The more solute, the fewer molecules of solvent in the gas phase and the lower the vapor pressure. Fewer molecules of solvent in gas phase above a solution Solution (solute plus solvent)

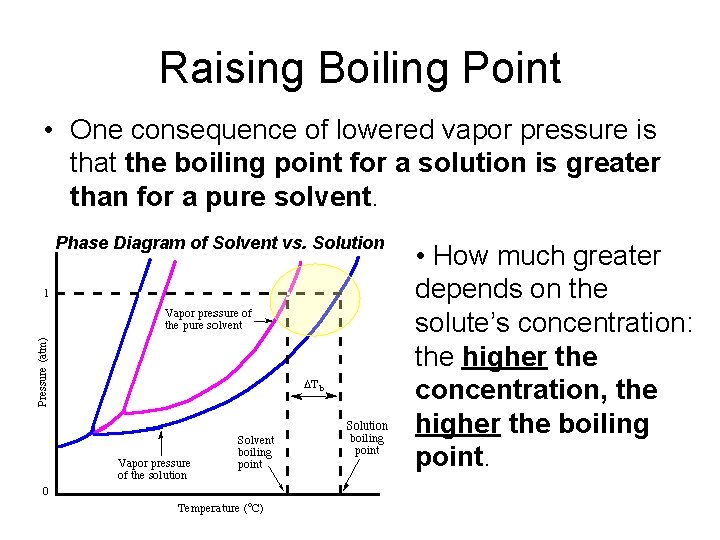
Raising Boiling Point • One consequence of lowered vapor pressure is that the boiling point for a solution is greater than for a pure solvent. Phase Diagram of Solvent vs. Solution • How much greater depends on the solute’s concentration: the higher the concentration, the higher the boiling point.

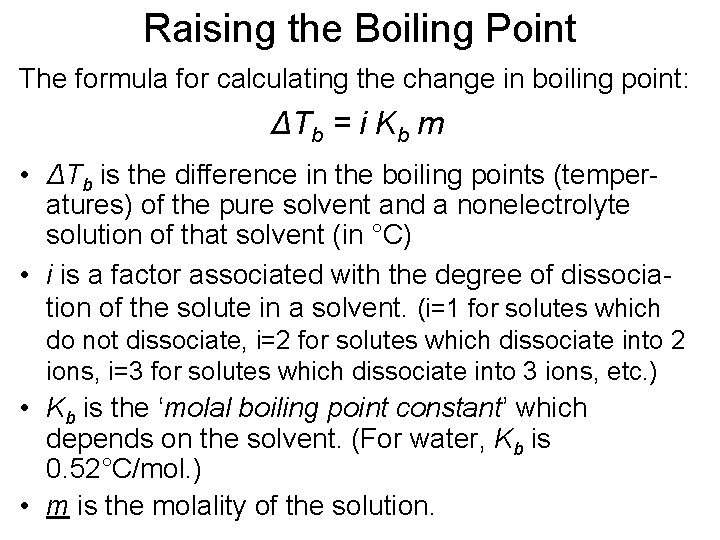
Raising the Boiling Point The formula for calculating the change in boiling point: ΔTb = i Kb m • ΔTb is the difference in the boiling points (temperatures) of the pure solvent and a nonelectrolyte solution of that solvent (in °C) • i is a factor associated with the degree of dissociation of the solute in a solvent. (i=1 for solutes which do not dissociate, i=2 for solutes which dissociate into 2 ions, i=3 for solutes which dissociate into 3 ions, etc. ) • Kb is the ‘molal boiling point constant’ which depends on the solvent. (For water, Kb is 0. 52°C/mol. ) • m is the molality of the solution.

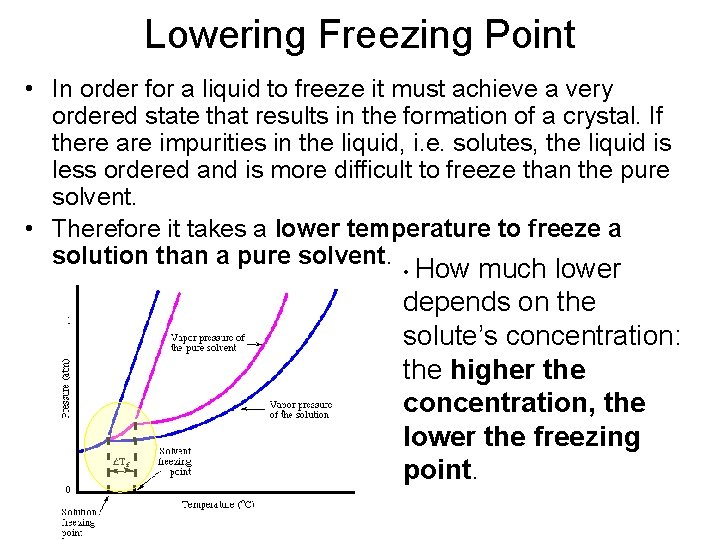
Lowering Freezing Point • In order for a liquid to freeze it must achieve a very ordered state that results in the formation of a crystal. If there are impurities in the liquid, i. e. solutes, the liquid is less ordered and is more difficult to freeze than the pure solvent. • Therefore it takes a lower temperature to freeze a solution than a pure solvent. • How much lower depends on the solute’s concentration: the higher the concentration, the lower the freezing point.

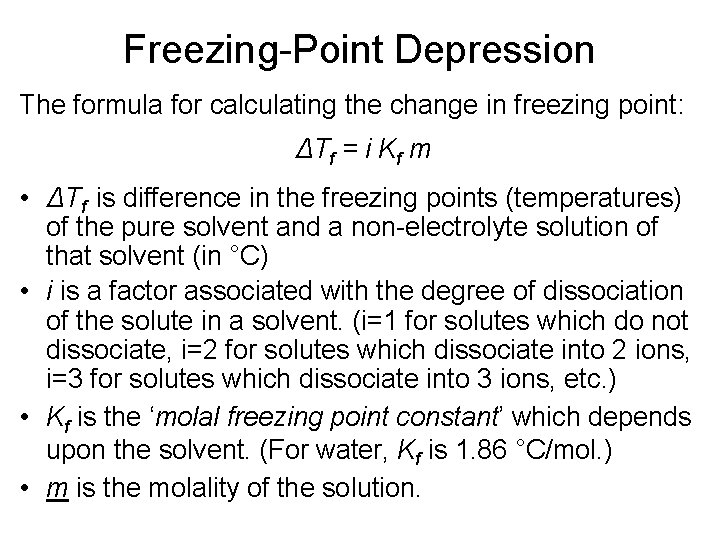
Freezing-Point Depression The formula for calculating the change in freezing point: ΔTf = i Kf m • ΔTf is difference in the freezing points (temperatures) of the pure solvent and a non-electrolyte solution of that solvent (in °C) • i is a factor associated with the degree of dissociation of the solute in a solvent. (i=1 for solutes which do not dissociate, i=2 for solutes which dissociate into 2 ions, i=3 for solutes which dissociate into 3 ions, etc. ) • Kf is the ‘molal freezing point constant’ which depends upon the solvent. (For water, Kf is 1. 86 °C/mol. ) • m is the molality of the solution.

Applications of Colligative Properties • Salting sidewalks and roads in the winter lowers the freezing point of the water on them, keeping them from icing up. • Adding antifreeze (ethylene glycol) to your car’s radiator system both lowers its freezing point in the winter and raises its boiling point in the summer, permitting a higher operating temperature range and protecting your radiator from boiling over.

Applications of Colligative Properties • Solutions typically do not freeze solidly. Pure solvent crystals freeze from the mixture, turning it slushy. • The solution that remains is more concentrated, dropping its freezing point even further. • Finally a matrix of frozen solid solvent with pockets of highly concentrated solution results.

Applications of Colligative Properties • A good example of a “frozen solution” is a popsicle. You know that it is not as solidly frozen as regular ice at freezer temps. • What happens if you suck on it instead of eating it a bit at a time? You get a very sweet liquid (concentrated solution) and a tasteless stick of water ice (the solvent matrix)!

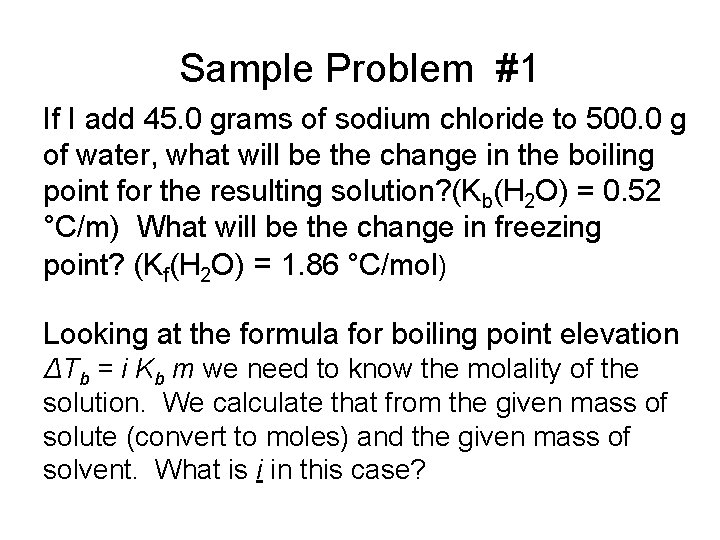
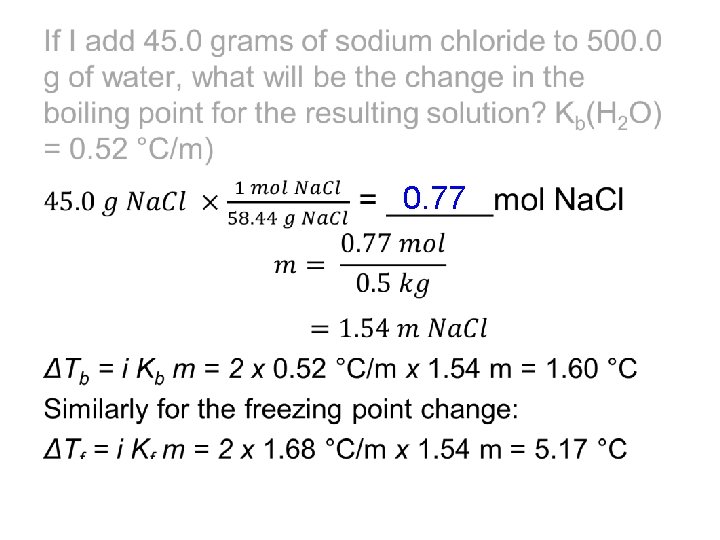
Sample Problem #1 If I add 45. 0 grams of sodium chloride to 500. 0 g of water, what will be the change in the boiling point for the resulting solution? (Kb(H 2 O) = 0. 52 °C/m) What will be the change in freezing point? (Kf(H 2 O) = 1. 86 °C/mol) Looking at the formula for boiling point elevation ΔTb = i Kb m we need to know the molality of the solution. We calculate that from the given mass of solute (convert to moles) and the given mass of solvent. What is i in this case?


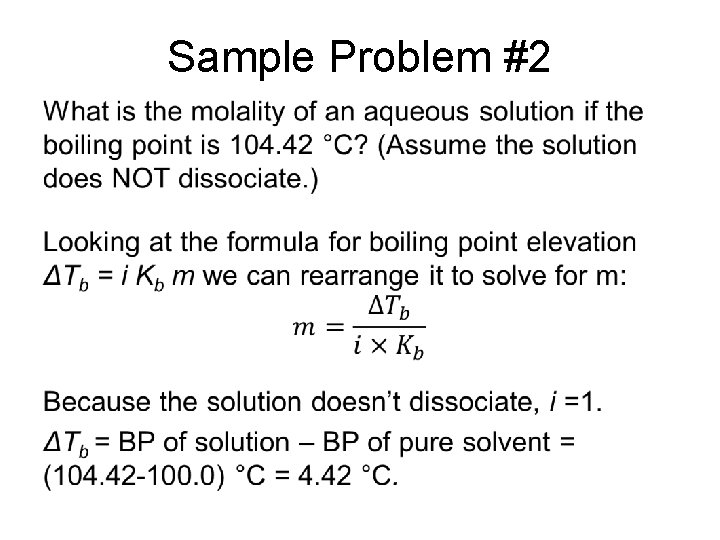
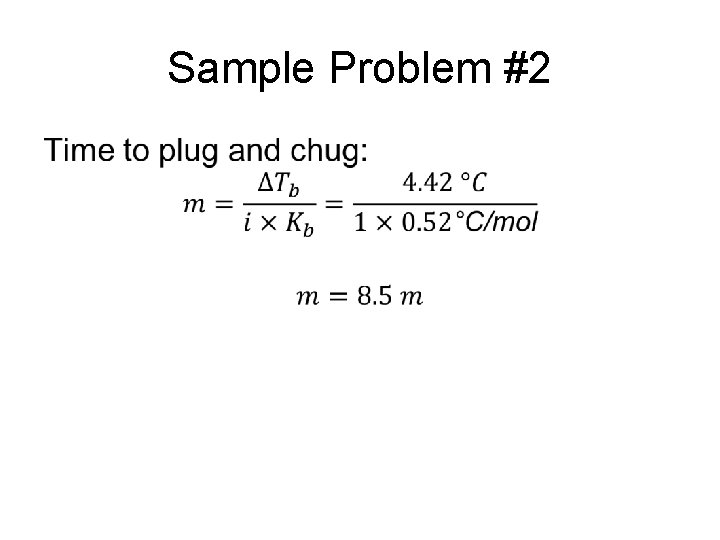
Sample Problem #2 •

Sample Problem #2 •