Ionospheres of the Terrestrial Planets Stan Solomon High










- Slides: 45

Ionospheres of the Terrestrial Planets Stan Solomon High Altitude Observatory National Center for Atmospheric Research stans@ucar. edu 1

Outline • Introduction to Earth’s ionosphere • Overview of Earth’s atmosphere • Ionization processes • Chemical processes • Mars & Venus • Why is Earth so different? 2

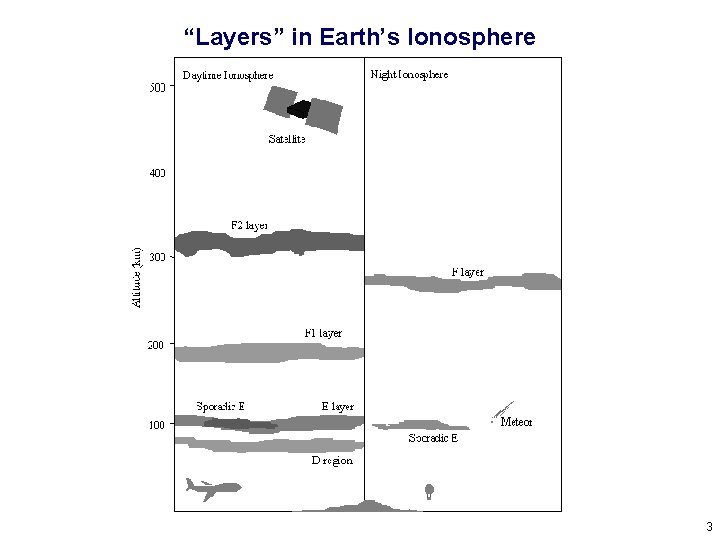
“Layers” in Earth’s Ionosphere 3

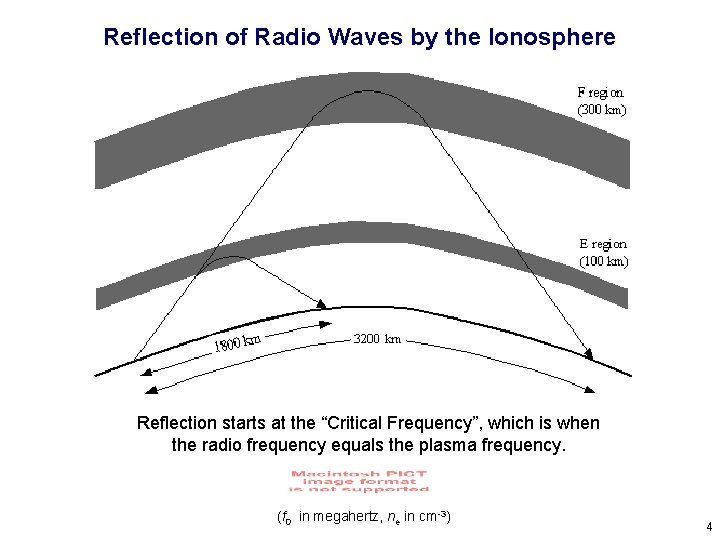
Reflection of Radio Waves by the Ionosphere Reflection starts at the “Critical Frequency”, which is when the radio frequency equals the plasma frequency. (f 0 in megahertz, ne in cm-3) 4

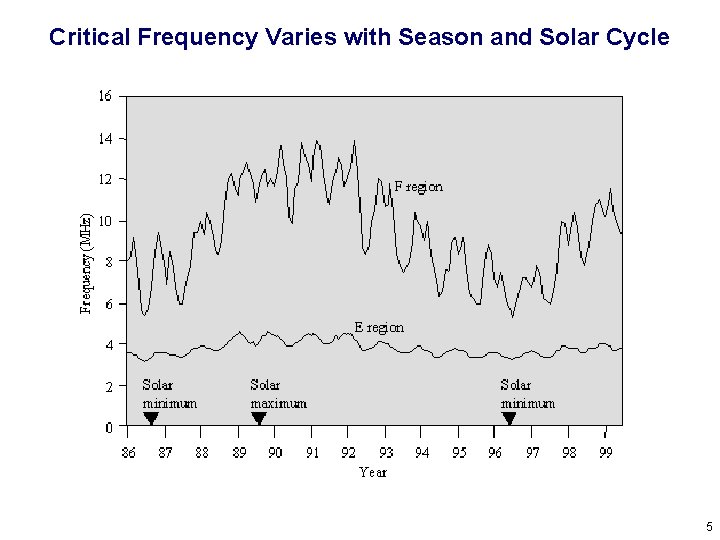
Critical Frequency Varies with Season and Solar Cycle 5

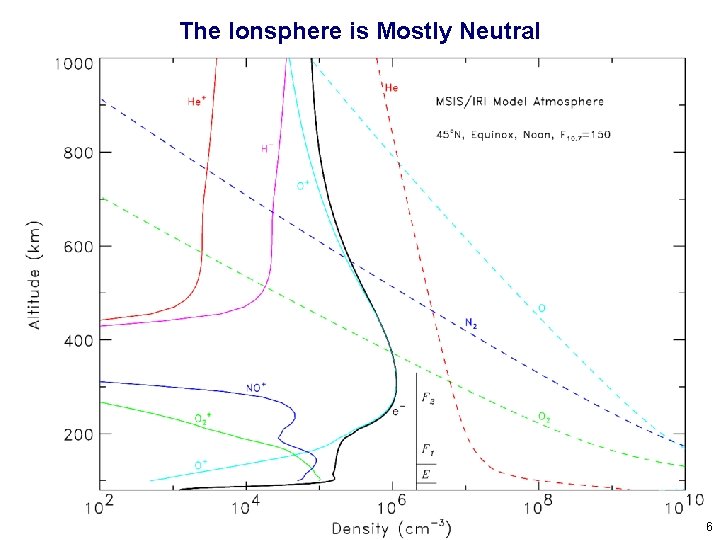
The Ionsphere is Mostly Neutral 6

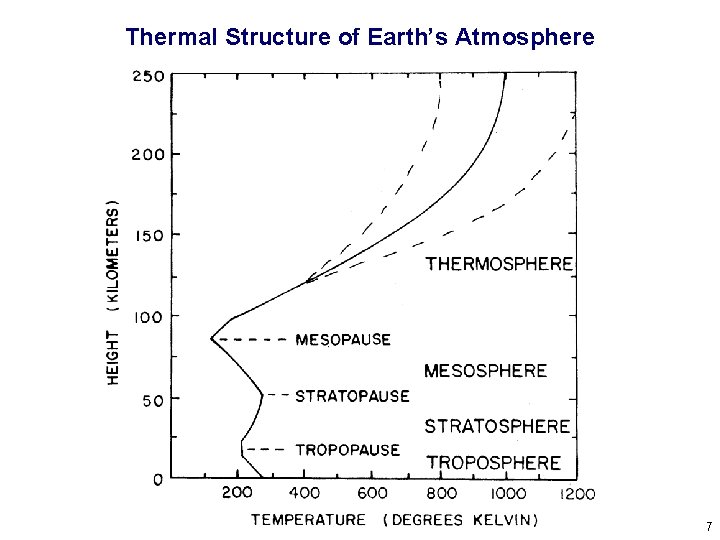
Thermal Structure of Earth’s Atmosphere 7

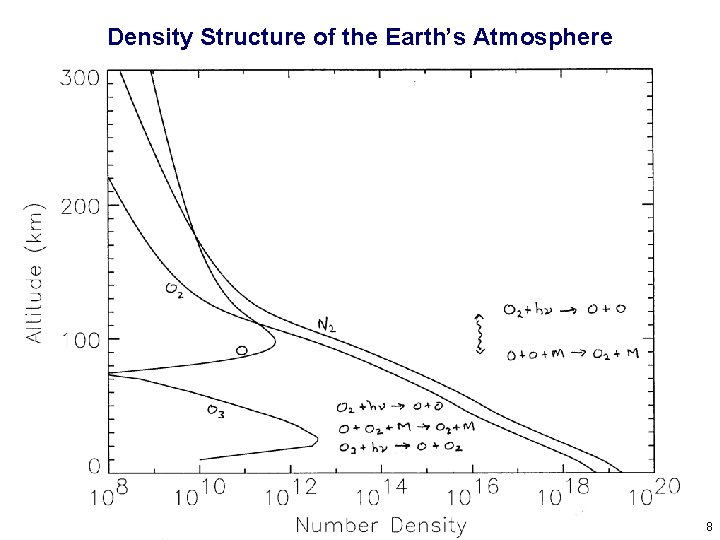
Density Structure of the Earth’s Atmosphere 8

Atmospheric Distribution in Hydrostatic Equilibrium 9

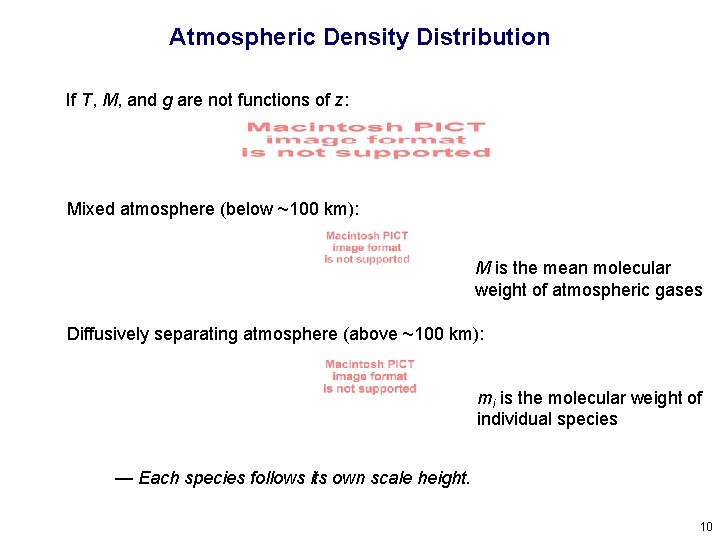
Atmospheric Density Distribution If T, M, and g are not functions of z: Mixed atmosphere (below ~100 km): M is the mean molecular weight of atmospheric gases Diffusively separating atmosphere (above ~100 km): mi is the molecular weight of individual species — Each species follows its own scale height. 10

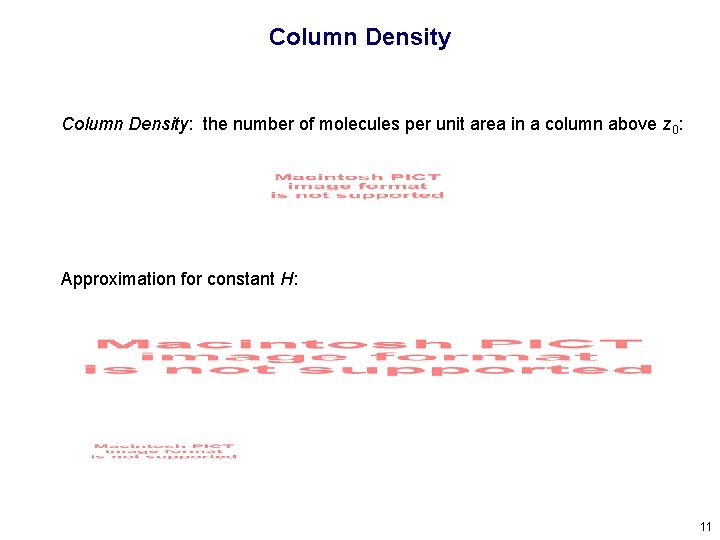
Column Density: the number of molecules per unit area in a column above z 0: Approximation for constant H: 11

Time Out to Think Suppose that a satellite is in low-Earth orbit at 300 km altitude. If the temperature of thermosphere increases (for instance, as a result of an increase in solar ultraviolet radiation) then the density at 300 km will: 1. Increase 2. Decrease 12

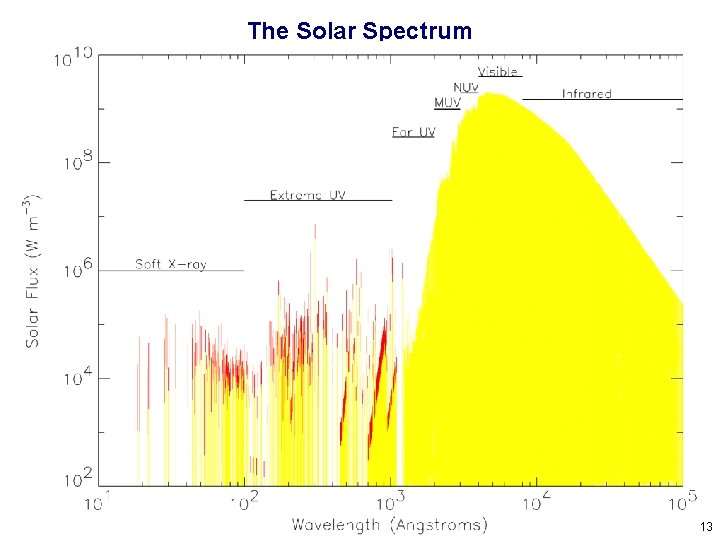
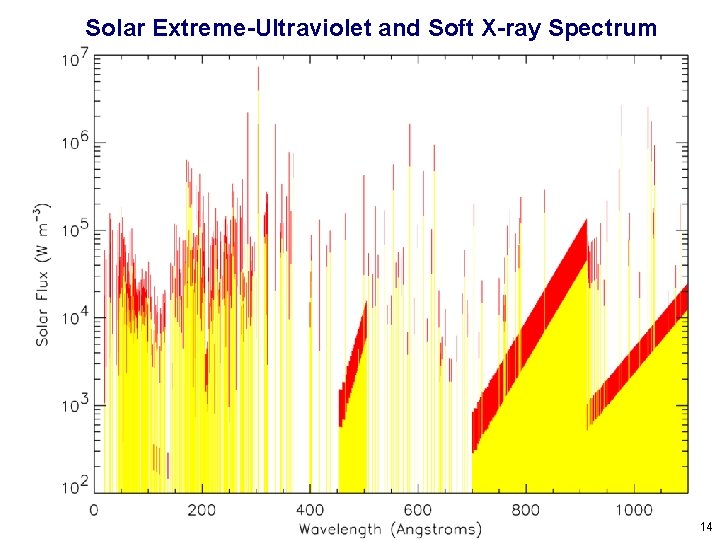
The Solar Spectrum 13

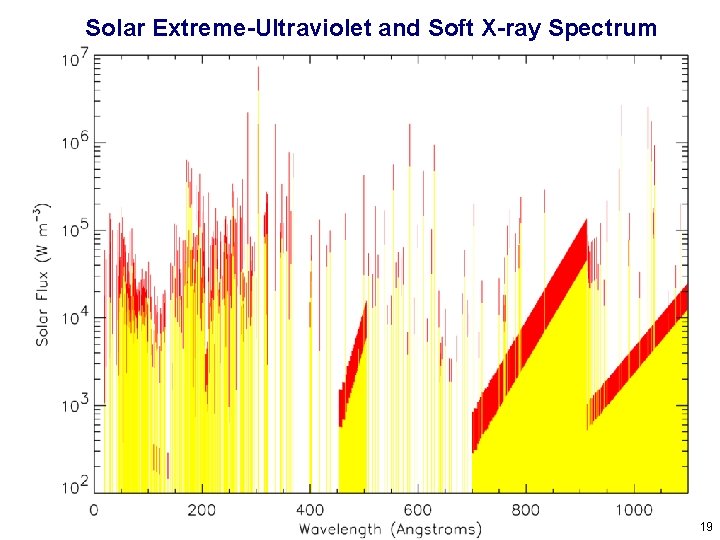
Solar Extreme-Ultraviolet and Soft X-ray Spectrum 14

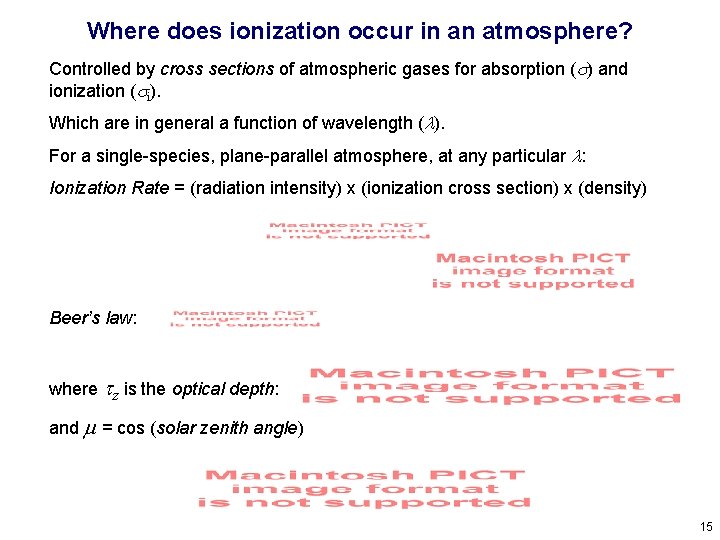
Where does ionization occur in an atmosphere? Controlled by cross sections of atmospheric gases for absorption (s) and ionization (si). Which are in general a function of wavelength (l). For a single-species, plane-parallel atmosphere, at any particular l: Ionization Rate = (radiation intensity) x (ionization cross section) x (density) Beer’s law: where tz is the optical depth: and m = cos (solar zenith angle) 15

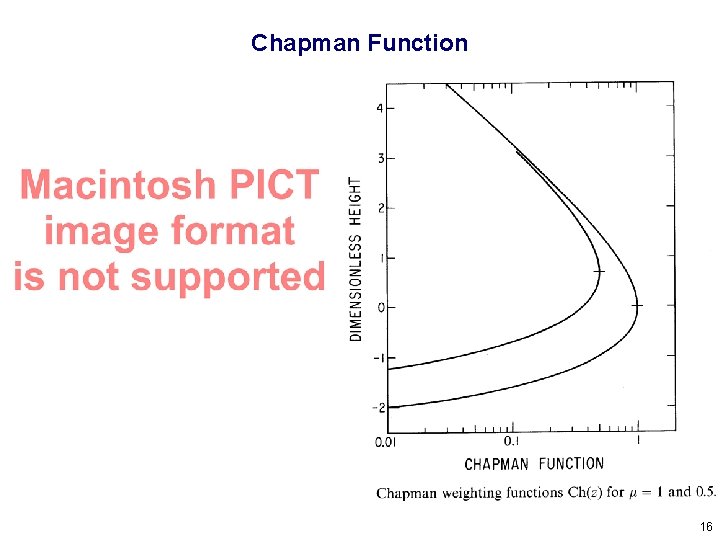
Chapman Function 16

Where is the peak of a Chapman function? 17

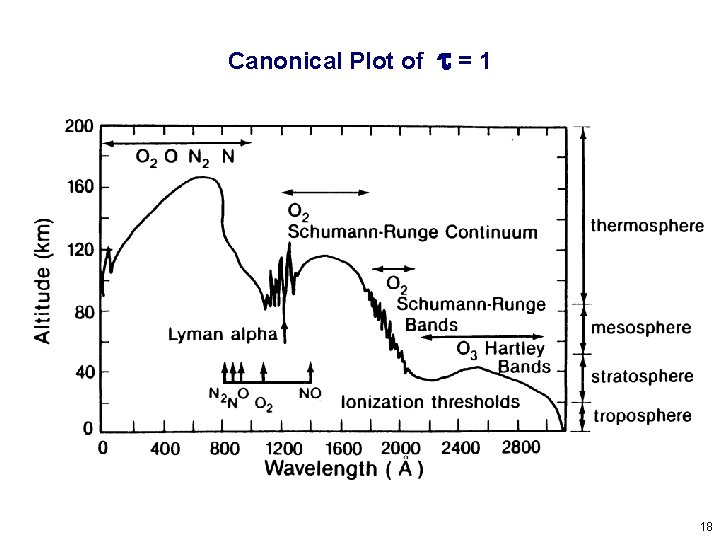
Canonical Plot of t=1 18

Solar Extreme-Ultraviolet and Soft X-ray Spectrum 19

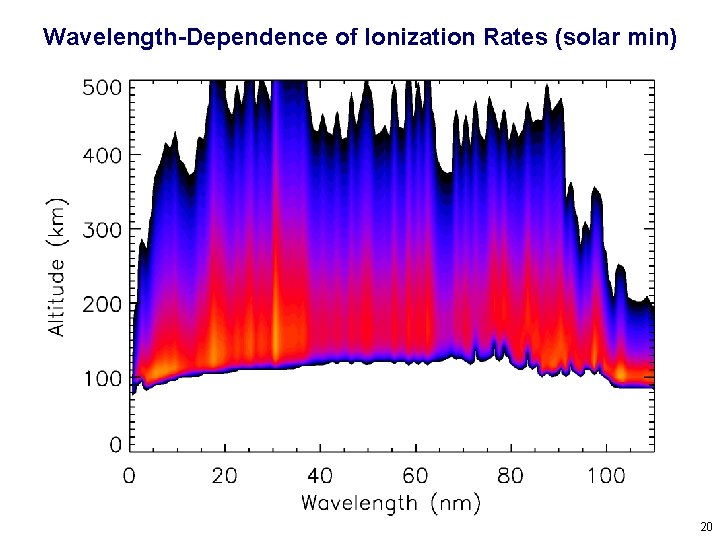
Wavelength-Dependence of Ionization Rates (solar min) 20

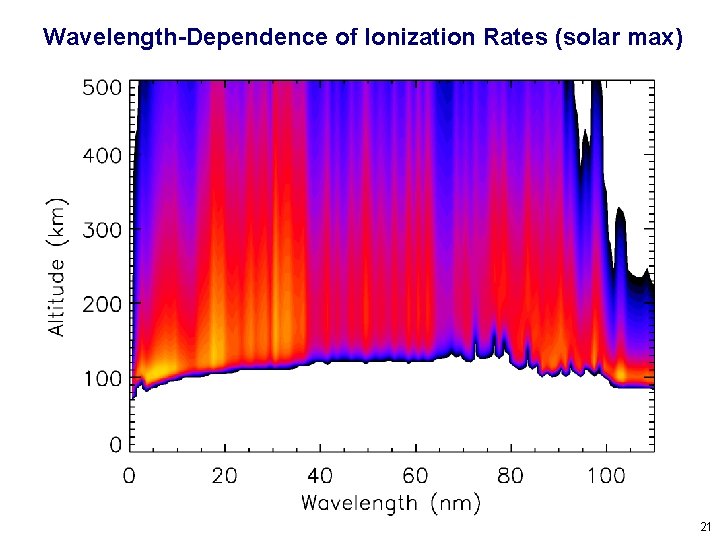
Wavelength-Dependence of Ionization Rates (solar max) 21

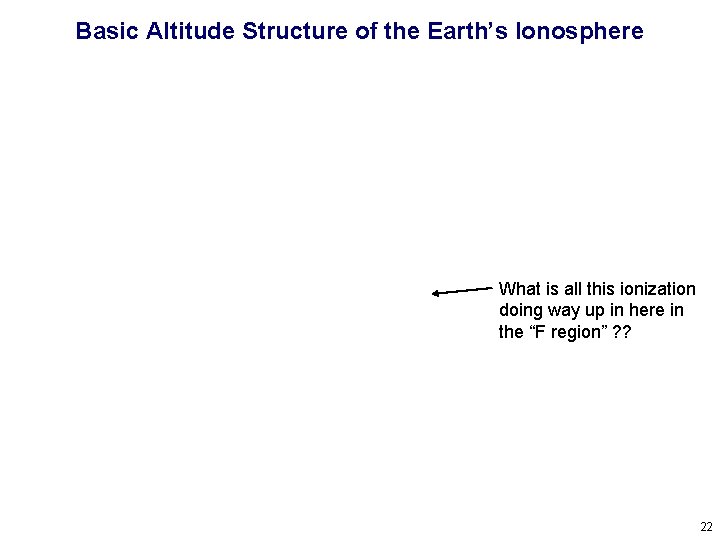
Basic Altitude Structure of the Earth’s Ionosphere What is all this ionization doing way up in here in the “F region” ? ? 22

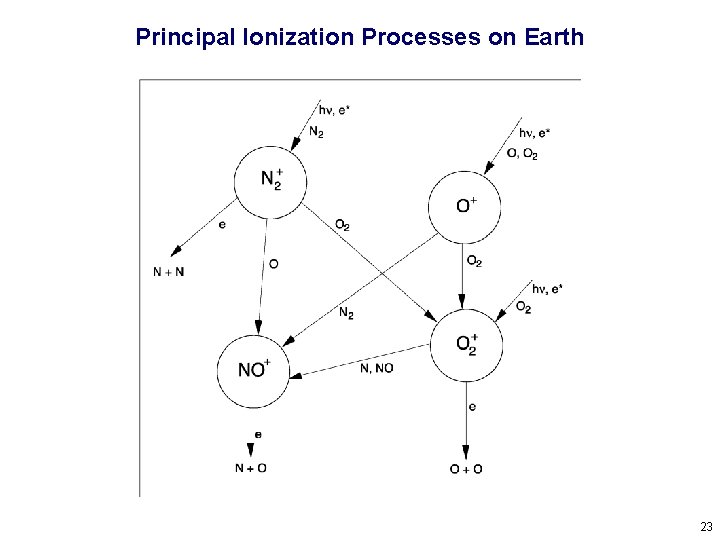
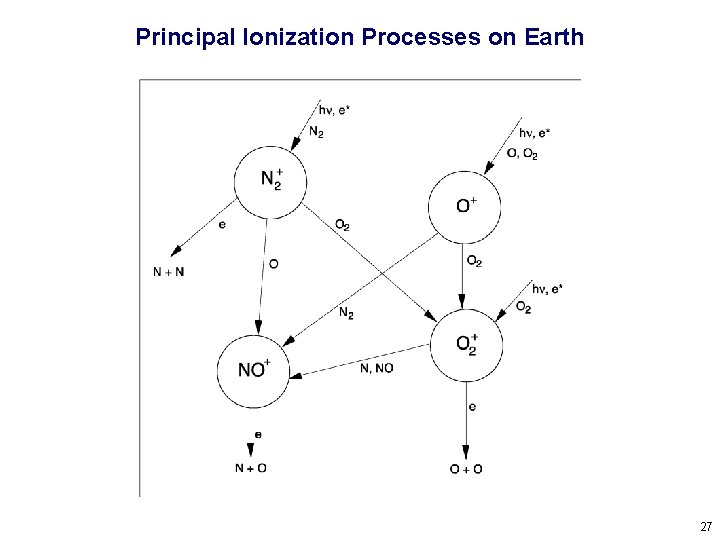
Principal Ionization Processes on Earth 23

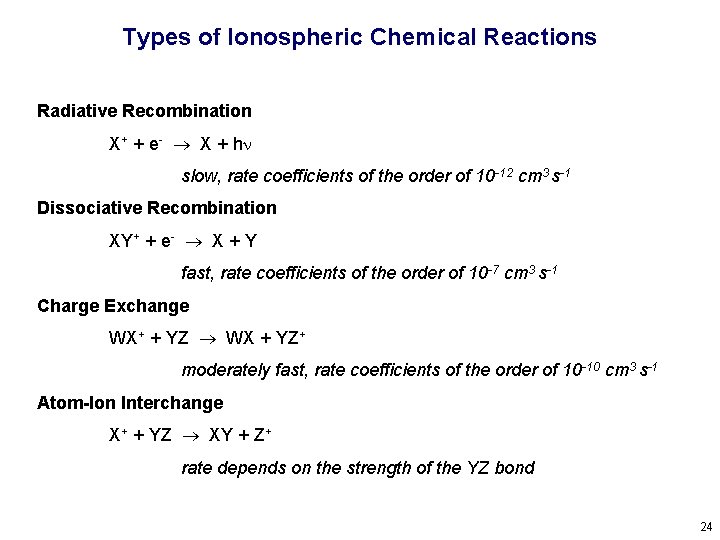
Types of Ionospheric Chemical Reactions Radiative Recombination X+ + e- X + hn slow, rate coefficients of the order of 10 -12 cm 3 s-1 Dissociative Recombination XY+ + e- X + Y fast, rate coefficients of the order of 10 -7 cm 3 s-1 Charge Exchange WX+ + YZ WX + YZ+ moderately fast, rate coefficients of the order of 10 -10 cm 3 s-1 Atom-Ion Interchange X+ + YZ XY + Z+ rate depends on the strength of the YZ bond 24

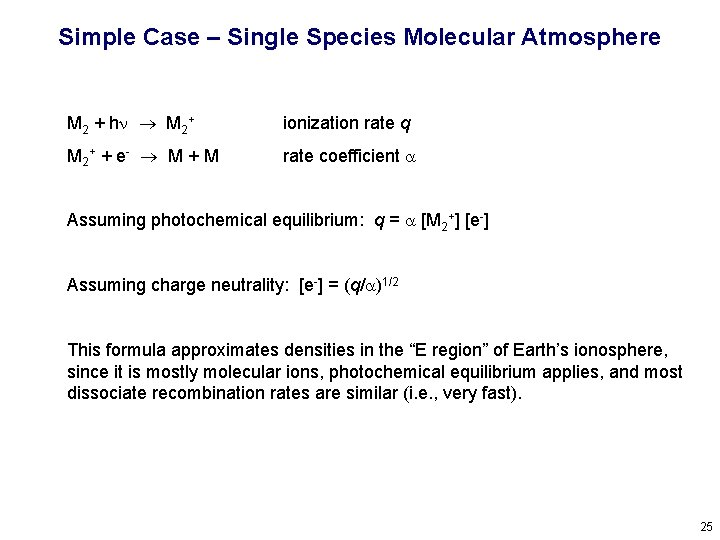
Simple Case – Single Species Molecular Atmosphere M 2 + hn M 2+ ionization rate q M 2 + + e - M + M rate coefficient a Assuming photochemical equilibrium: q = a [M 2+] [e-] Assuming charge neutrality: [e-] = (q/a)1/2 This formula approximates densities in the “E region” of Earth’s ionosphere, since it is mostly molecular ions, photochemical equilibrium applies, and most dissociate recombination rates are similar (i. e. , very fast). 25

Complicated Case – Earth’s F-Region Ionosphere Because of the decrease in molecular densities, the photochemical lifetime of O+ becomes longer than the diffusion lifetime (time it takes to move a scale height in the vertical direction) above ~200 km. Thus, the F region is not a simple Chapman layer caused by the absorption of radiation, but rather a balance between chemical production at lower altitude and ambipolar diffusion at higher altitude. The long lifetime of O+ at high altitude is also why the F 2 region persists at night. 26

Principal Ionization Processes on Earth 27

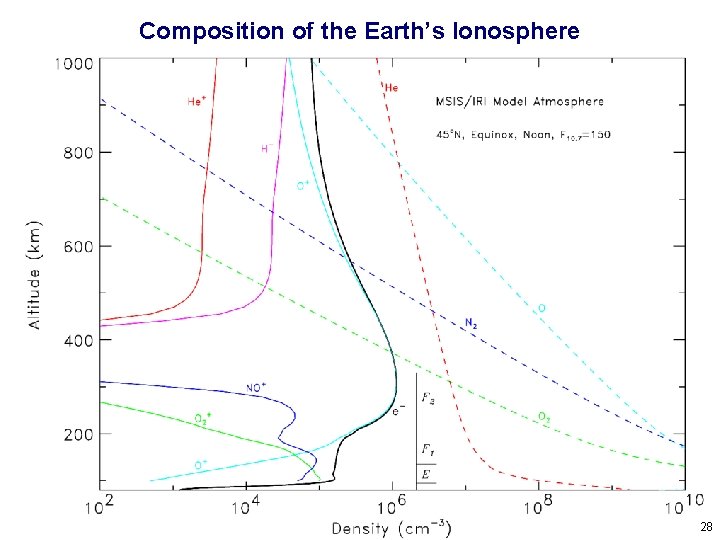
Composition of the Earth’s Ionosphere 28

Ionospheric Electrodynamics 29

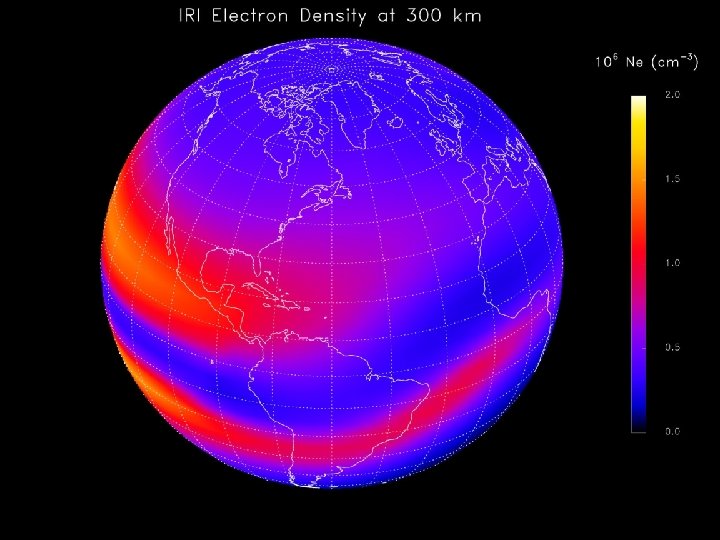
International Reference Ionosphere at 300 km 30

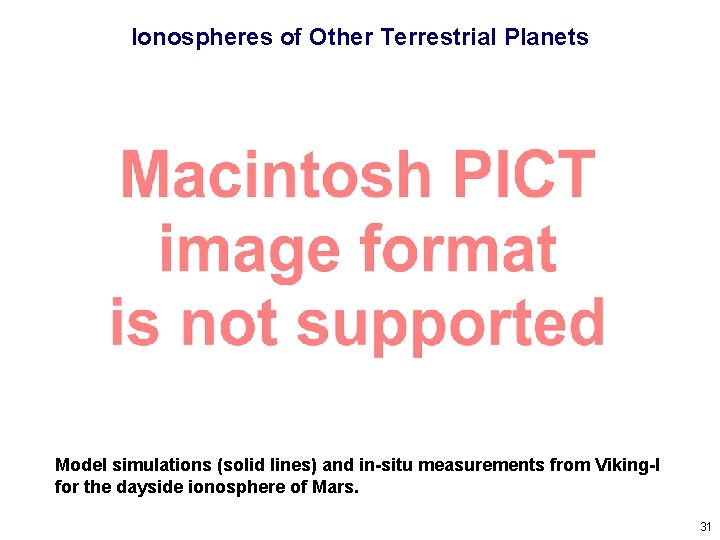
Ionospheres of Other Terrestrial Planets Model simulations (solid lines) and in-situ measurements from Viking-I for the dayside ionosphere of Mars. 31

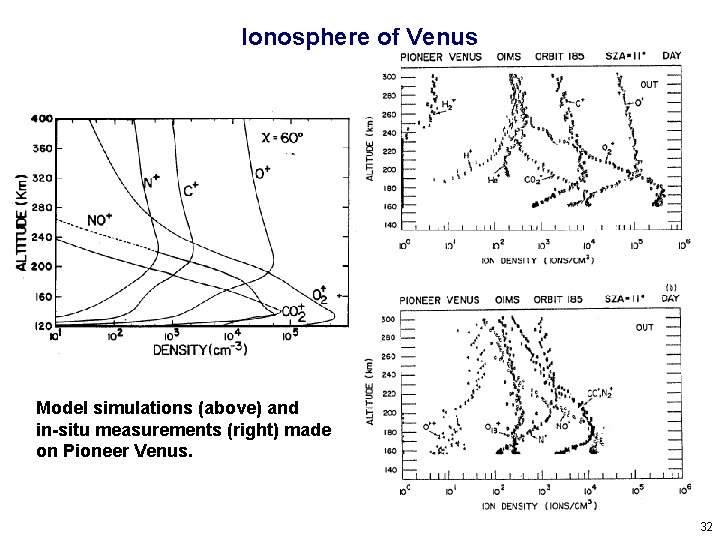
Ionosphere of Venus Model simulations (above) and in-situ measurements (right) made on Pioneer Venus. 32

Why are the ionospheres of Mars and Venus, although similar to each other, so different from Earth? N 2+, O 2+ and O+ are the most abundantly produced ions in Earth’s ionosphere because N 2, O 2 and O are the most abundant neutral species in the lower part of thermosphere. However, the most abundant ions below 300 km are O+, NO+, and O 2 + On Mars and Venus the most abundantly produced ions are CO 2+ and O+, but the most abundant ions are O 2+ and O+. Unlike Earth, there is no “F 2 region”, and very little ionization at night. — Why doesn’t O+ have a longer lifetime on Mars and Venus? — Why is there so much O 2+ when there’s so little O 2 in their atmospheres? 33

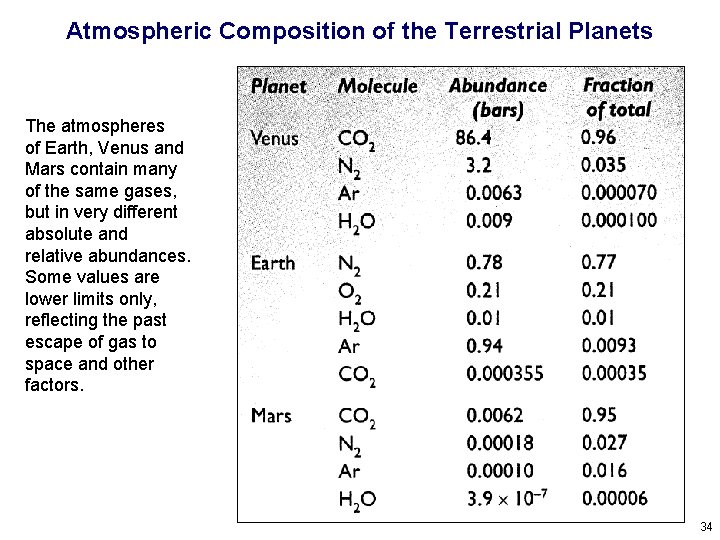
Atmospheric Composition of the Terrestrial Planets The atmospheres of Earth, Venus and Mars contain many of the same gases, but in very different absolute and relative abundances. Some values are lower limits only, reflecting the past escape of gas to space and other factors. 34

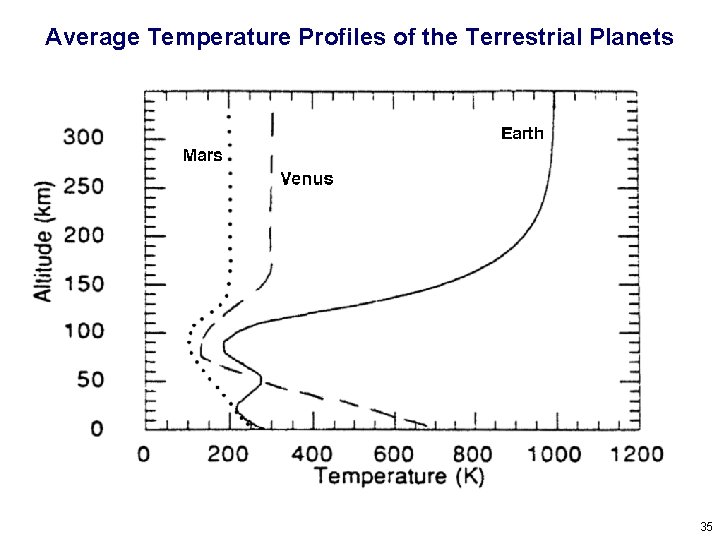
Average Temperature Profiles of the Terrestrial Planets 35

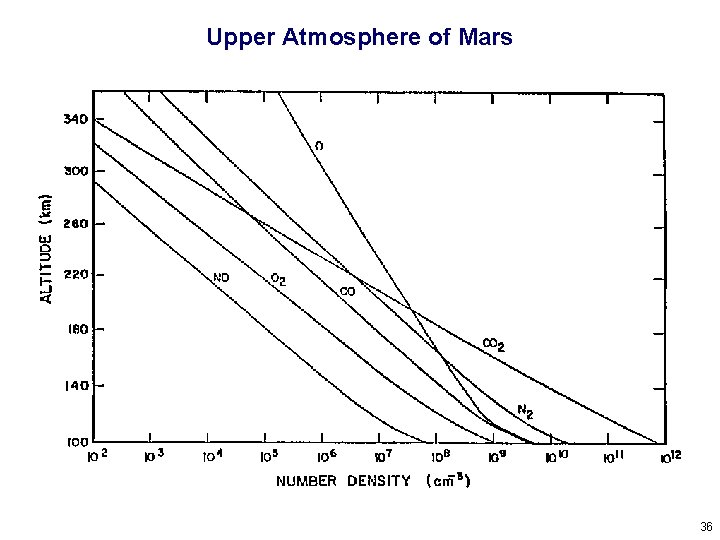
Upper Atmosphere of Mars 36

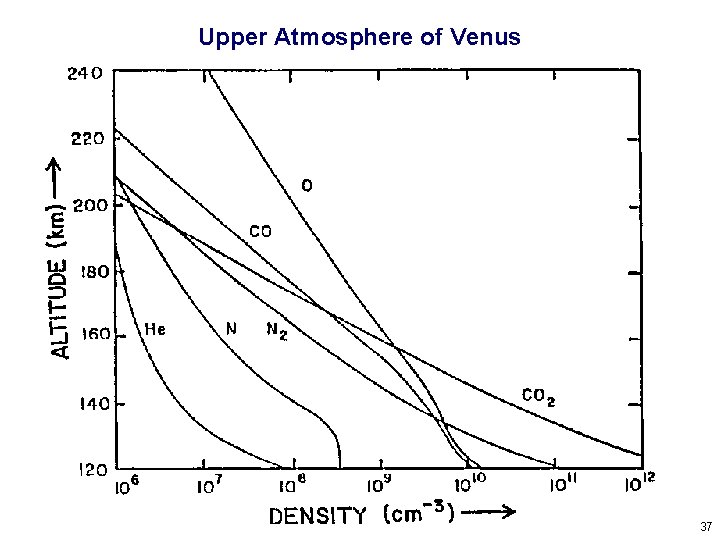
Upper Atmosphere of Venus 37

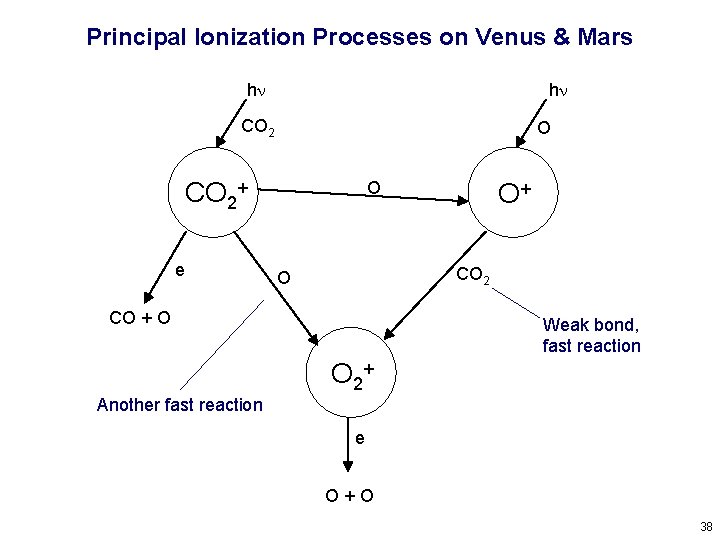
Principal Ionization Processes on Venus & Mars hn hn CO 2 O CO 2+ e O O+ CO 2 O CO + O Weak bond, fast reaction O 2+ Another fast reaction e O+O 38

Venus and Mars are “Normal”, Earth is Anomalous On Venus and Mars, O+ reacts rapidly with CO 2 and CO 2+ reacts rapidly with O because these atom-ion interchange reactions have fast rate coefficients. This is because CO 2 is not very strongly bonded, compared to N 2. Therefore, Venus and Mars ionospheres are “E region” (or “F 1 region”) types, controlled mostly by photochemical equilibrium at their peaks. Earth lacks sufficient carbon in its atmosphere, and doesn’t have enough O 2 at high altitude, for this to happen. Atom-ion interchange of O+ with N 2 is very slow, due to the strength of the N 2 bond. This creates the high, dense, persistant “F 2 region” and a lot of interesting ionospheric variability. 39

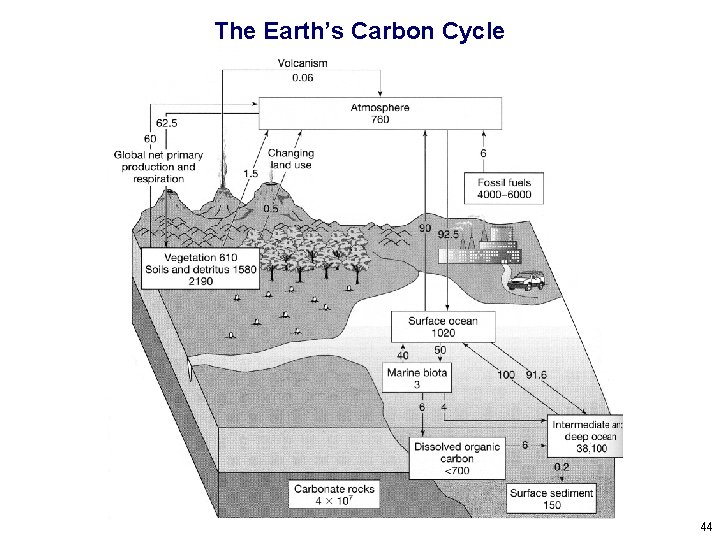
So… Where’s the Carbon? 40

Genesse River, Letchworth State Park, N. Y. 41

Middle Falls 42

White Cliffs of Dover 43

The Earth’s Carbon Cycle 44

Time Out to Think A high, dense, “F 2 layer” ionosphere observed on a terrestrial-type planet would be a sign of life on that planet. 1. True 2. False 45
 What are jovian planets made of
What are jovian planets made of Terrestrial planets surface
Terrestrial planets surface What are the inner and outer planets
What are the inner and outer planets Inner terrestrial planets
Inner terrestrial planets Terrestrial planets
Terrestrial planets Stan solomon
Stan solomon What separates the inner planets and outer planets
What separates the inner planets and outer planets The first four outer planets are
The first four outer planets are What separates inner and outer planets
What separates inner and outer planets Terrestrial habitat
Terrestrial habitat Food chain in land
Food chain in land Pond succession
Pond succession Chapter 3 section 2 terrestrial biomes
Chapter 3 section 2 terrestrial biomes Mercury diameter km
Mercury diameter km Terrestrial production system
Terrestrial production system Terrestrial coordinate system
Terrestrial coordinate system Impacts of wildlife trade on terrestrial biodiversity
Impacts of wildlife trade on terrestrial biodiversity Umts network architecture
Umts network architecture Infosolar
Infosolar Are protists terrestrial or aquatic
Are protists terrestrial or aquatic Human impact on terrestrial ecosystems
Human impact on terrestrial ecosystems What is terrestrial navigation?
What is terrestrial navigation? Terrestrial life
Terrestrial life Solar terrestrial relations observatory
Solar terrestrial relations observatory Food web analysis worksheet
Food web analysis worksheet Terrestrial only
Terrestrial only Respiration in terrestrial animals
Respiration in terrestrial animals Hydroponics is the science of growing terrestrial plants in
Hydroponics is the science of growing terrestrial plants in My very excited mother just
My very excited mother just Terrestrial biomes summary chart
Terrestrial biomes summary chart Photosynthetic, multicellular, and terrestrial?
Photosynthetic, multicellular, and terrestrial? Terrestrial soil
Terrestrial soil Terrestrial animals
Terrestrial animals Gymnosperms
Gymnosperms Food chain labeled
Food chain labeled Are bacteria autotrophs or heterotrophs
Are bacteria autotrophs or heterotrophs Terrestrial biome examples
Terrestrial biome examples Westheide
Westheide Aquatic vs terrestrial
Aquatic vs terrestrial Taiga climatograph
Taiga climatograph A terrestrial food web
A terrestrial food web đại từ thay thế
đại từ thay thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Công thức tiính động năng
Công thức tiính động năng Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay