Unit 4 Nutrient Cycles in Marine Ecosystems AICE









- Slides: 30

Unit 4 - Nutrient Cycles in Marine Ecosystems AICE Marine

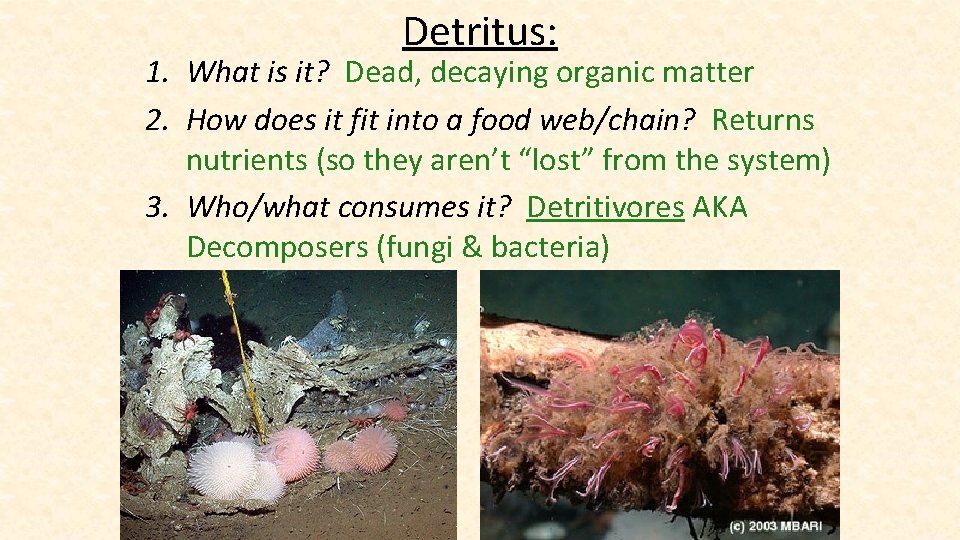
Detritus: 1. What is it? 2. How does it fit in to a food web/chain? 3. Who/what consumes it?

Detritus: 1. What is it? Dead, decaying organic matter 2. How does it fit into a food web/chain? Returns nutrients (so they aren’t “lost” from the system) 3. Who/what consumes it? Detritivores AKA Decomposers (fungi & bacteria)

• Detritus is broken down and released as chemical elements into the soil, water, & air where P. P. can recycle them into organic compounds • Without detritivores, life would cease! Essential nutrients would be locked up & unavailable to the ecosystem


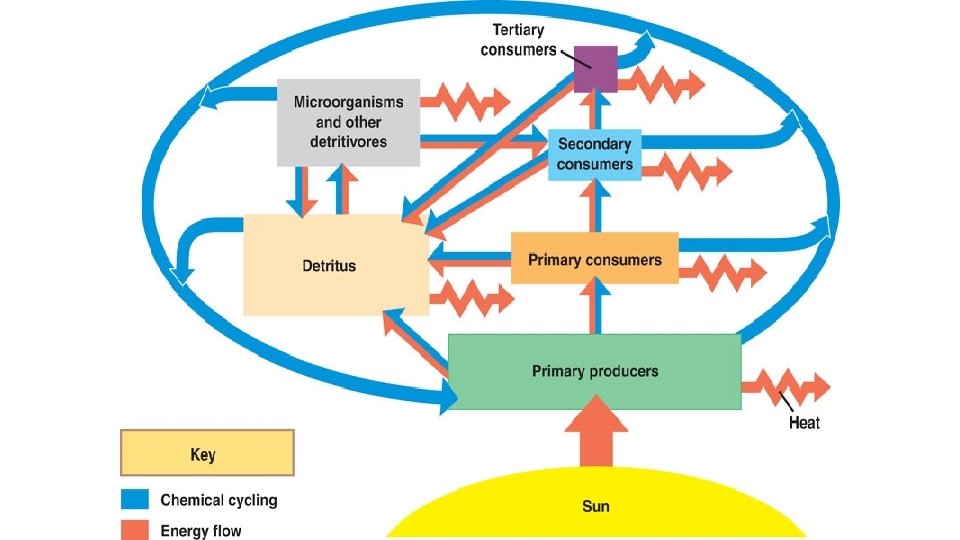
Chemical Cycling: • Nutrients move between organic and inorganic parts of the ecosystem in biogeochemical cycles. • Cycles may be global or local. Nutrient cycles with a gaseous component (carbon, sulfur, nitrogen) are global whereas phosphorus, potassium and calcium cycle more locally (at least on short time scales).

2 Major Types of Biogeochemical Cycles: **Based on the Primary Source of nutrient input** 1. Gaseous cycles—atmosphere & ocean (global circulation patterns) 2. Sedimentary cycles—soil & rocks of the Earth’s crust; these vary from one element to another, but essentially each has two abiotic phases: a) Salt Solution phase b) Rock phase

Draw me! Fig. 1

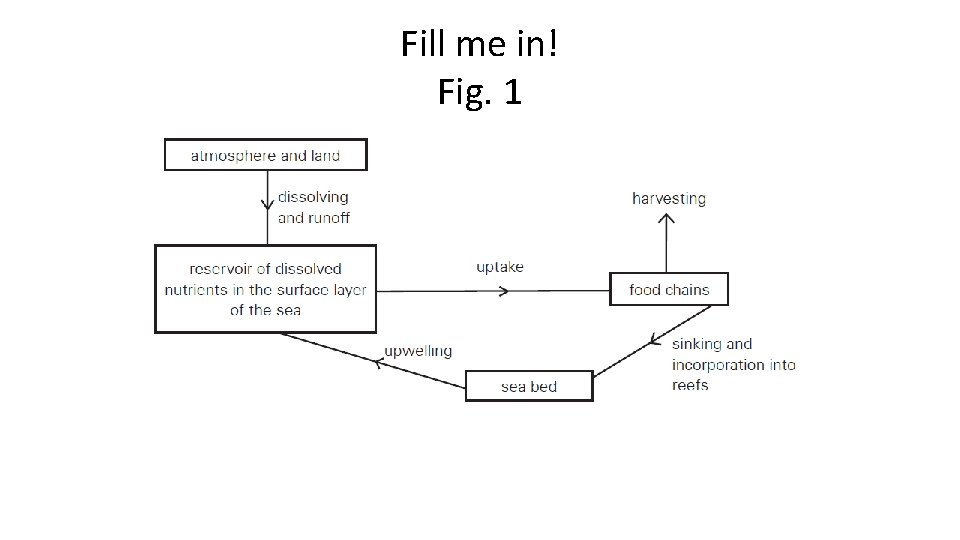
Fill me in! Fig. 1

General Structure of Nutrient Cycles: • Nutrients can move from one reservoir to another by a variety of processes • Some examples: – Photosynthesis (makes organic nutrients available from inorganic) – Respiration (makes inorganic nutrients available from organic) – Decomposition (makes inorganic nutrients available from organic) – Excretion (makes inorganic nutrients available from organic)

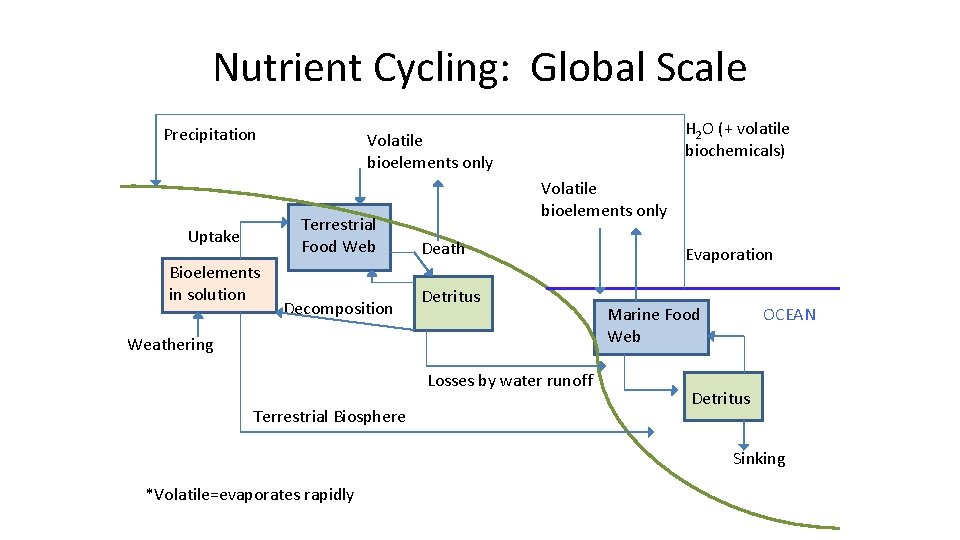
Nutrient Cycling: Global Scale Precipitation Terrestrial Food Web Uptake Bioelements in solution H 2 O (+ volatile biochemicals) Volatile bioelements only Decomposition Volatile bioelements only Death Detritus Weathering Losses by water runoff Terrestrial Biosphere Evaporation OCEAN Marine Food Web Detritus Sinking *Volatile=evaporates rapidly

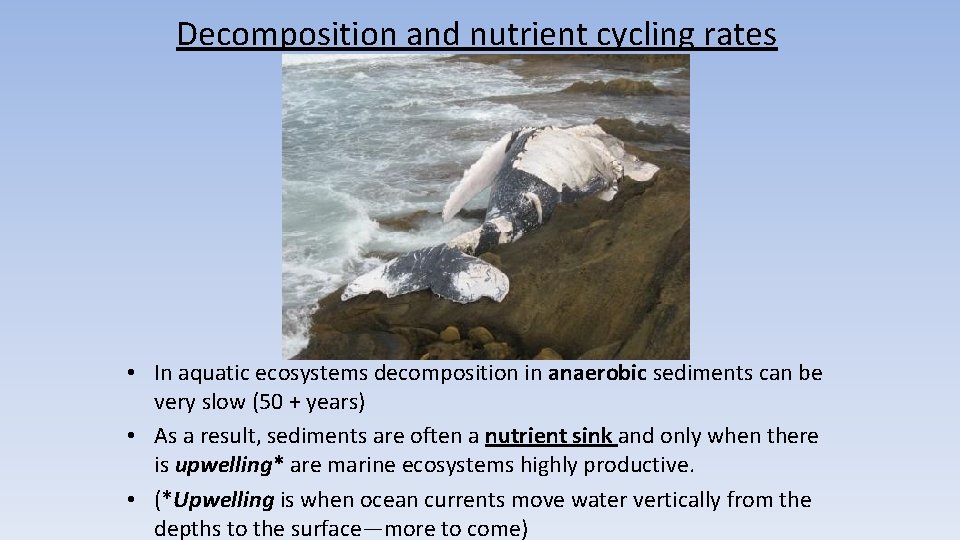
Decomposition and nutrient cycling rates • Rate of nutrient cycling is affected by rate of decomposition • In the tropics, warmer temperatures cause organic material to decompose 2 -3 X faster than it does in temperate regions!

Decomposition and nutrient cycling rates • In aquatic ecosystems decomposition in anaerobic sediments can be very slow (50 + years) • As a result, sediments are often a nutrient sink and only when there is upwelling* are marine ecosystems highly productive. • (*Upwelling is when ocean currents move water vertically from the depths to the surface—more to come)

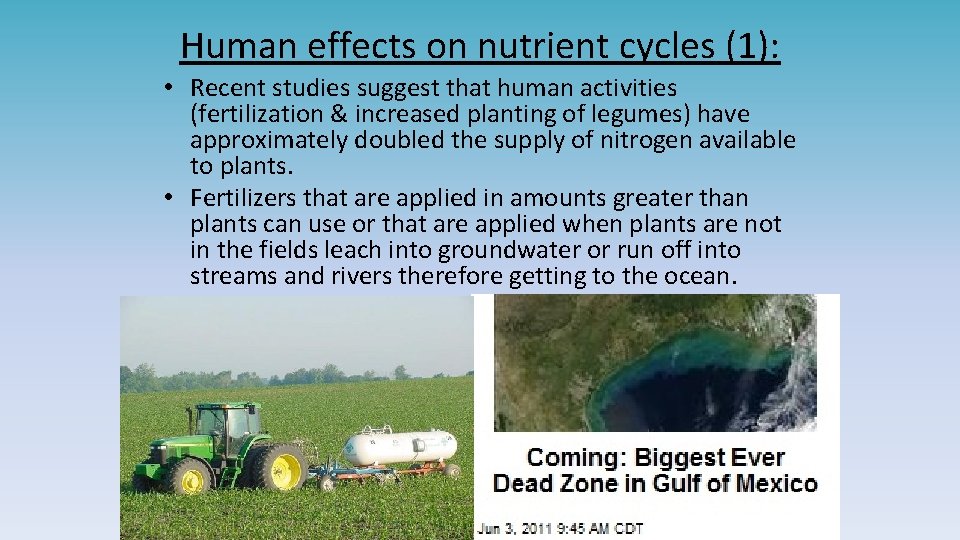
Human effects on nutrient cycles (1): • Recent studies suggest that human activities (fertilization & increased planting of legumes) have approximately doubled the supply of nitrogen available to plants. • Fertilizers that are applied in amounts greater than plants can use or that are applied when plants are not in the fields leach into groundwater or run off into streams and rivers therefore getting to the ocean.

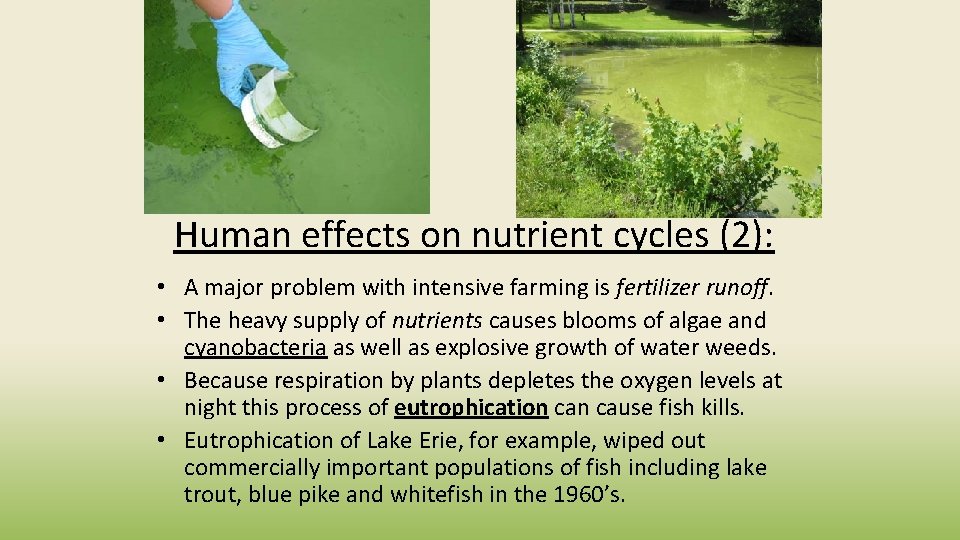
Human effects on nutrient cycles (2): • A major problem with intensive farming is fertilizer runoff. • The heavy supply of nutrients causes blooms of algae and cyanobacteria as well as explosive growth of water weeds. • Because respiration by plants depletes the oxygen levels at night this process of eutrophication cause fish kills. • Eutrophication of Lake Erie, for example, wiped out commercially important populations of fish including lake trout, blue pike and whitefish in the 1960’s.

Limits on primary productivity • Nutrient limitation is a major factor affecting NPP in aquatic biomes. • In marine environments the nutrients limiting primary productivity are usually nitrogen and phosphorus, which are scarce in the photic zone.

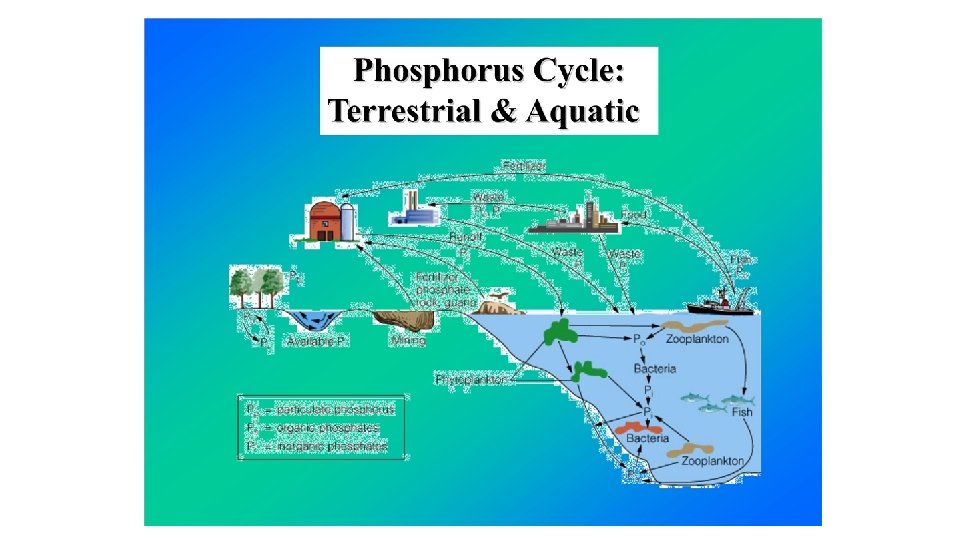
5 Marine Nutrient Cycles: 1. Carbon 2. Nitrogen 3. Phosphorus 4. Magnesium 5. Calcium

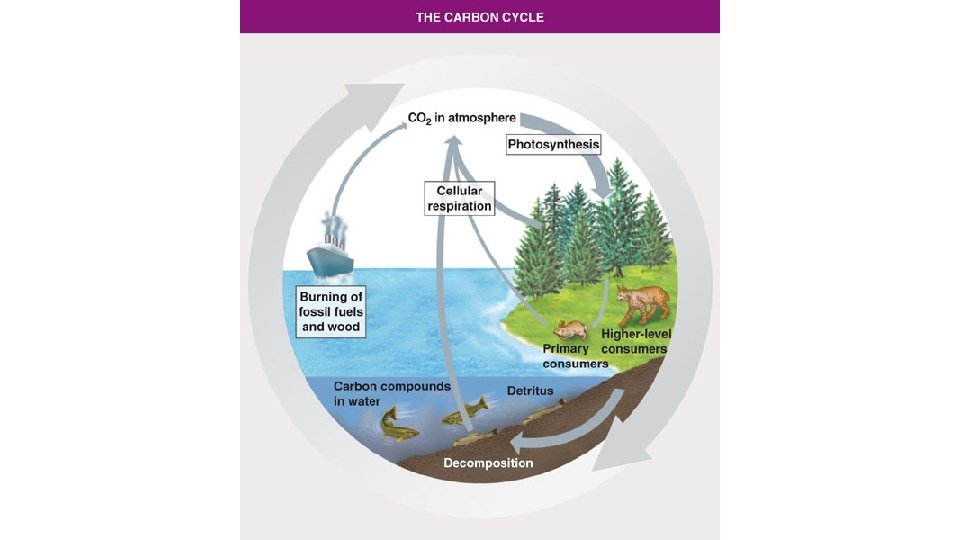
Carbon Cycle • Carbon forms the basis of all organic molecules (organic chemistry is the study of carbon chemistry). Fats, sugars, DNA, proteins etc. all contain carbon. • Photosynthetic organisms take in CO 2 from the air and using energy from sunlight join carbon atoms to make sugars (energy is stored in the chemical bonds).

Carbon Cycle • Major reservoirs of carbon include: atmospheric CO 2, ocean (dissolved carbon compounds), biomass of organisms, fossil fuels and sedimentary rocks.

Carbon Cycle • Photosynthesis removes large amounts of atmospheric CO 2 • An approximately equal amount of CO 2 is returned to atmosphere by cellular respiration. • Burning of fossil fuels adds large amounts CO 2 to the atmosphere.


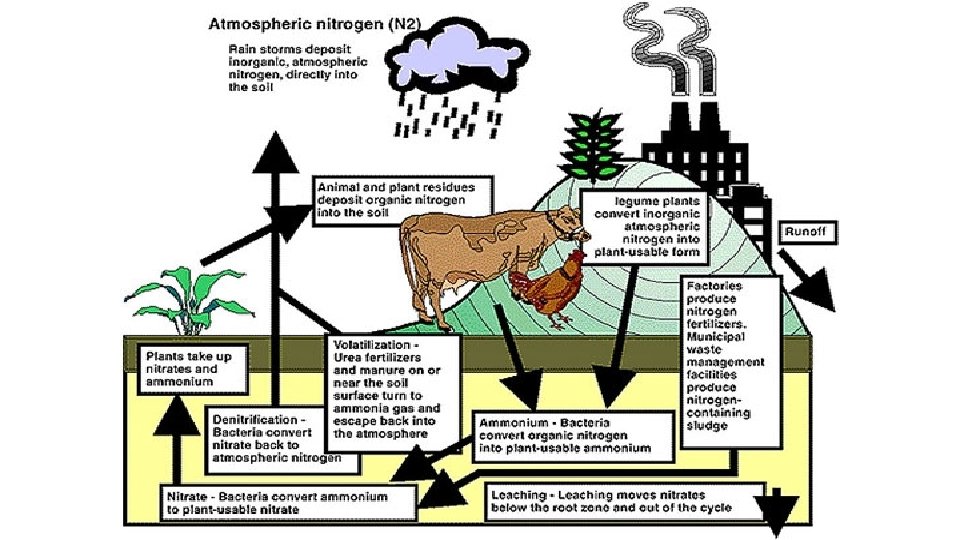
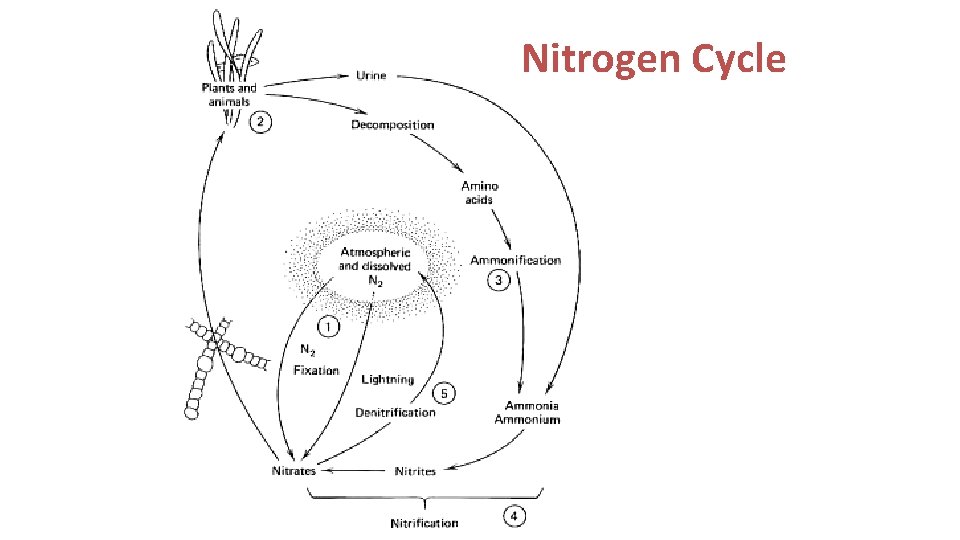
Nitrogen Cycling • N is an essential element in – Protein: each amino acid has at least 1 N – Nucleic acids: nitrogenous bases • N 2 makes up about 79% of the atmosphere • N 2 is only usable by prokaryotes with the enzyme nitrogenase – Cyanobacteria like Anabaena carry their nitrogenase in a low oxygen package, the heterocyst.

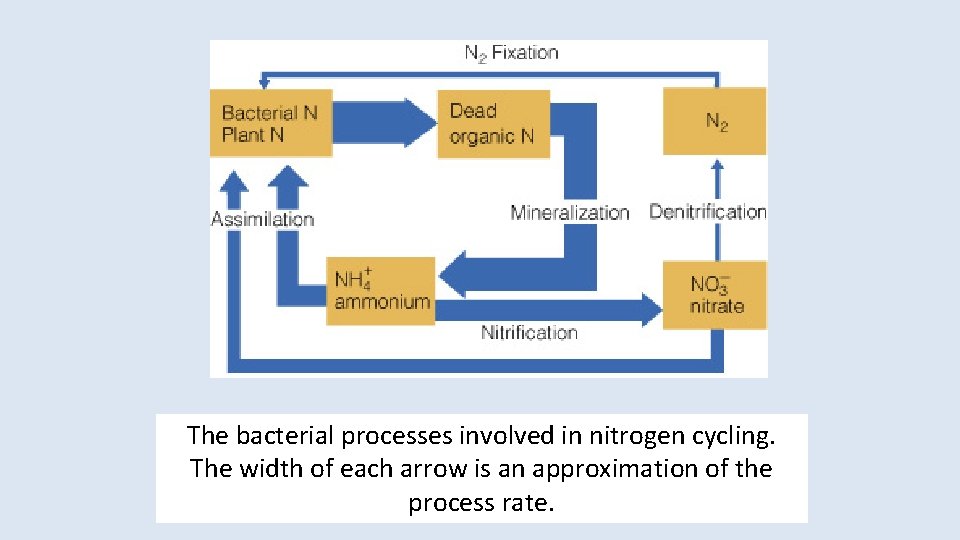
The bacterial processes involved in nitrogen cycling. The width of each arrow is an approximation of the process rate.


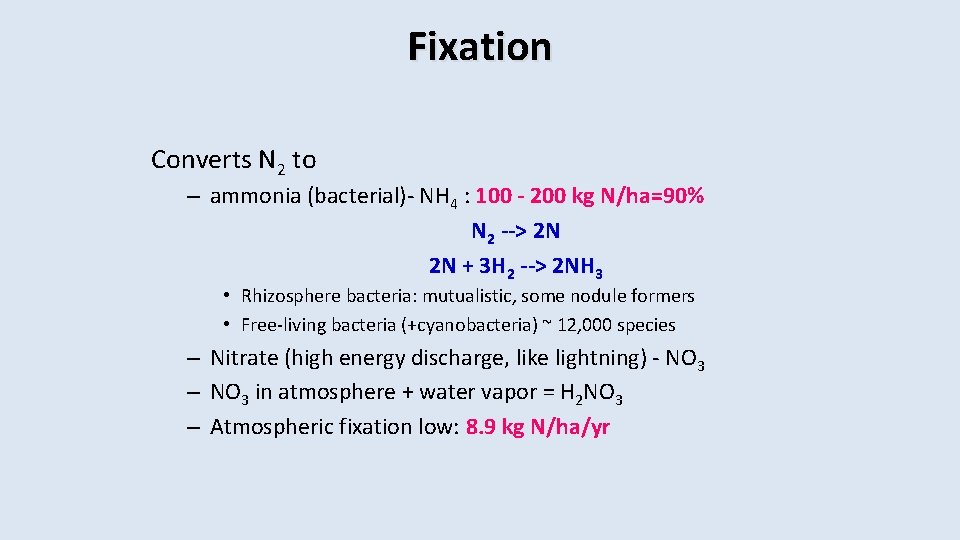
Fixation Converts N 2 to – ammonia (bacterial)- NH 4 : 100 - 200 kg N/ha=90% N 2 --> 2 N 2 N + 3 H 2 --> 2 NH 3 • Rhizosphere bacteria: mutualistic, some nodule formers • Free-living bacteria (+cyanobacteria) ~ 12, 000 species – Nitrate (high energy discharge, like lightning) - NO 3 – NO 3 in atmosphere + water vapor = H 2 NO 3 – Atmospheric fixation low: 8. 9 kg N/ha/yr

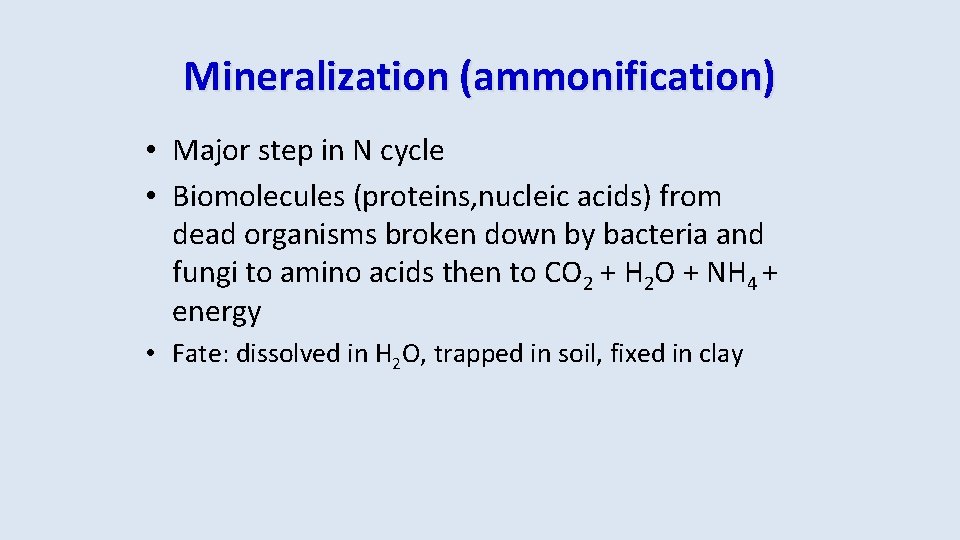
Mineralization (ammonification) • Major step in N cycle • Biomolecules (proteins, nucleic acids) from dead organisms broken down by bacteria and fungi to amino acids then to CO 2 + H 2 O + NH 4 + energy • Fate: dissolved in H 2 O, trapped in soil, fixed in clay

Nitrification • Nitrosomonas bacteria use ammonia in soil as sole E source NH 3 + 11/2 O 2 -> HNO 2 + H 2 + 165 kcal HNO 2 -> H+ + NO 2 • Nitrobacteria use the remaining E in nitrite ion and oxidize it to nitrate NO 2 + 1/2 O 2 -> NO 3 • Pollution can result if nitrates build up in aquatic ecosystems

Denitrification • Nitrates reduced biologically to N 2 • Denitrifiers are facultative anaerobes – Bacteria: Pseudomonas – Fungi

Nitrogen Cycle
