Nutrient cycles Ecosphere Photo Earth Photo Nutrient cycles








- Slides: 27


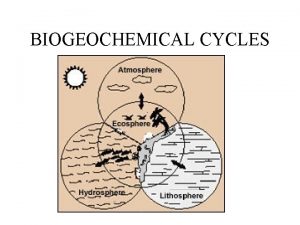
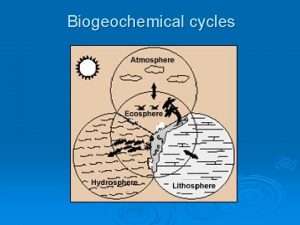
Nutrient cycles

Ecosphere Photo

Earth Photo

Nutrient cycles • Nutrient cycles, or “biogeochemical cycles, ” involve natural processes that recycle nutrients in various chemical forms in a cyclic manner from the nonliving environment to living organisms and back to the non-living environment again • Types of nutrient cycles: – Hydrologic cycle – Atmospheric cycles – Sedimentary cycles

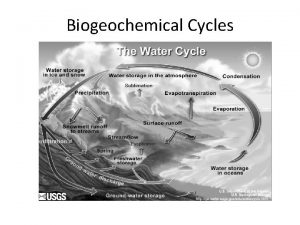
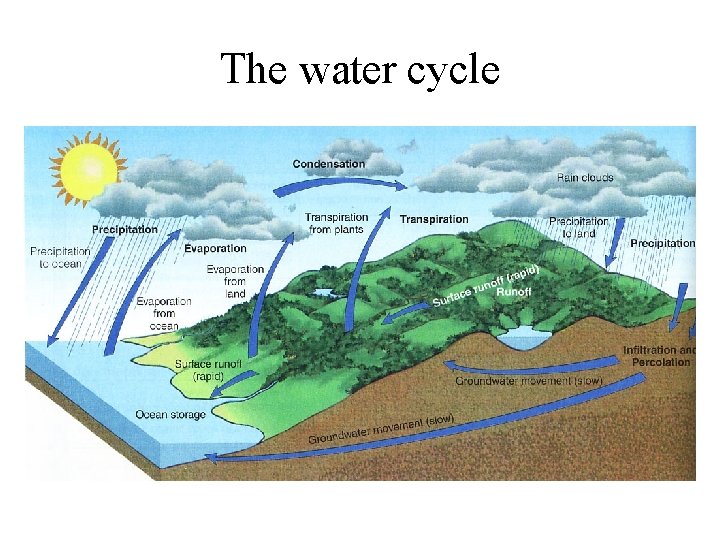
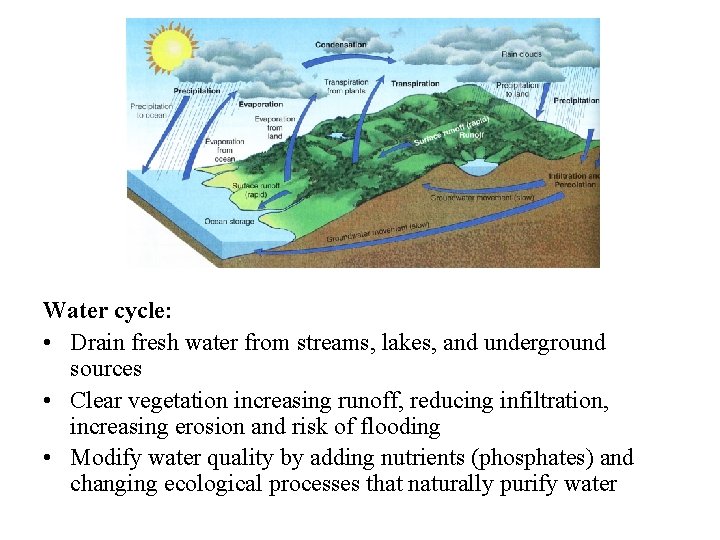
The water cycle

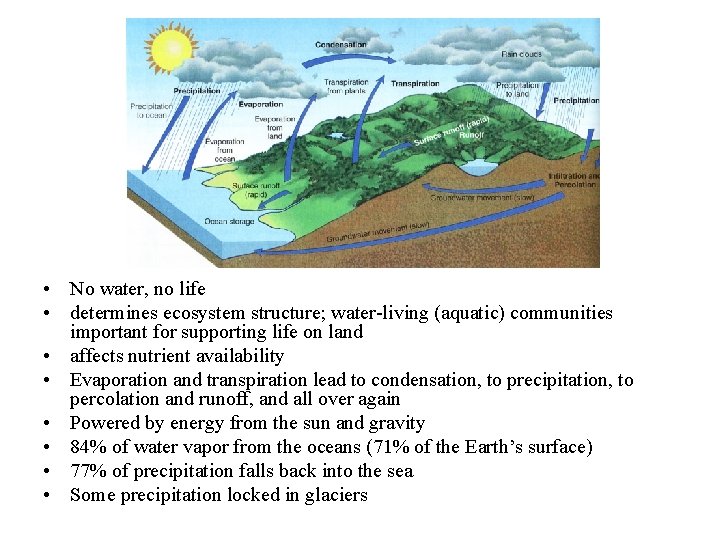
• No water, no life • determines ecosystem structure; water-living (aquatic) communities important for supporting life on land • affects nutrient availability • Evaporation and transpiration lead to condensation, to precipitation, to percolation and runoff, and all over again • Powered by energy from the sun and gravity • 84% of water vapor from the oceans (71% of the Earth’s surface) • 77% of precipitation falls back into the sea • Some precipitation locked in glaciers

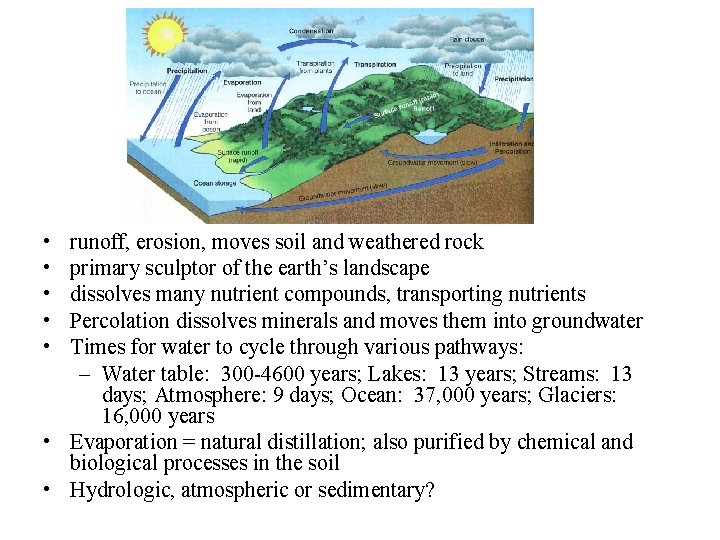
• • • runoff, erosion, moves soil and weathered rock primary sculptor of the earth’s landscape dissolves many nutrient compounds, transporting nutrients Percolation dissolves minerals and moves them into groundwater Times for water to cycle through various pathways: – Water table: 300 -4600 years; Lakes: 13 years; Streams: 13 days; Atmosphere: 9 days; Ocean: 37, 000 years; Glaciers: 16, 000 years • Evaporation = natural distillation; also purified by chemical and biological processes in the soil • Hydrologic, atmospheric or sedimentary?

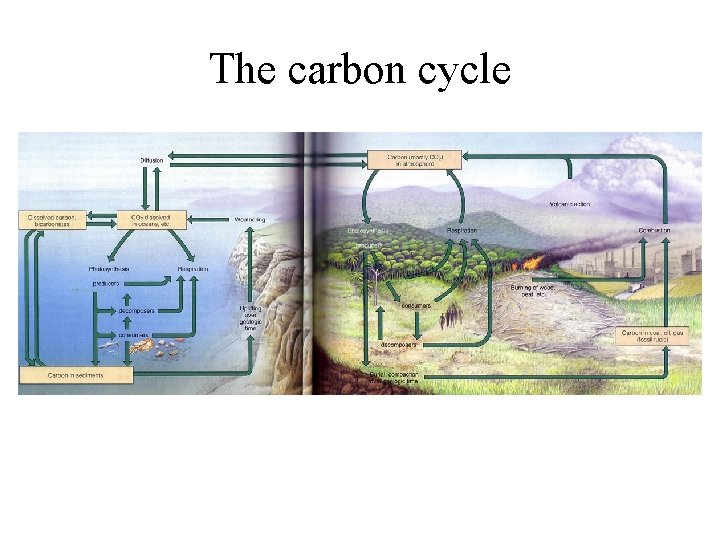
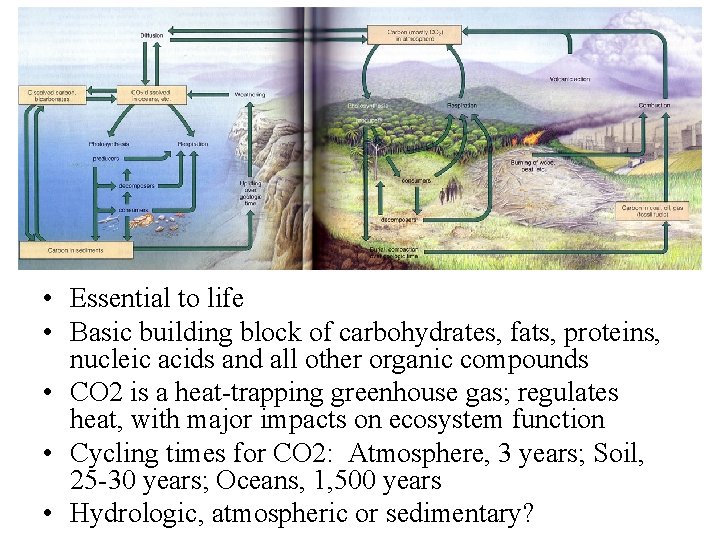
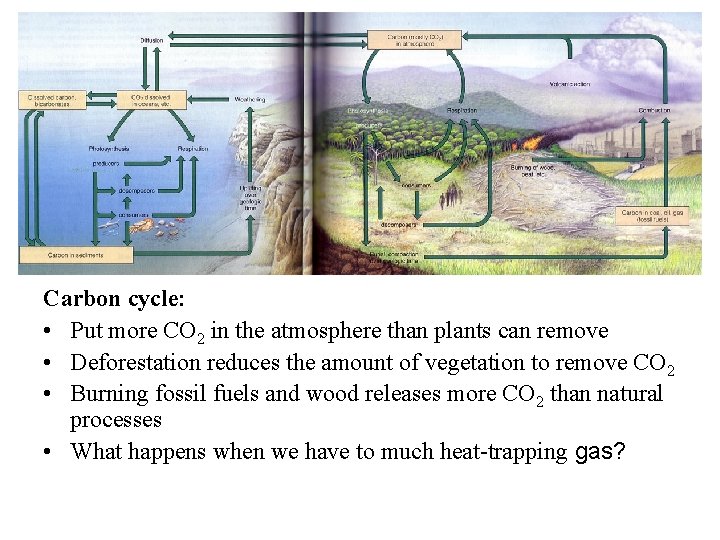
The carbon cycle

• Essential to life • Basic building block of carbohydrates, fats, proteins, nucleic acids and all other organic compounds • CO 2 is a heat-trapping greenhouse gas; regulates heat, with major impacts on ecosystem function • Cycling times for CO 2: Atmosphere, 3 years; Soil, 25 -30 years; Oceans, 1, 500 years • Hydrologic, atmospheric or sedimentary?

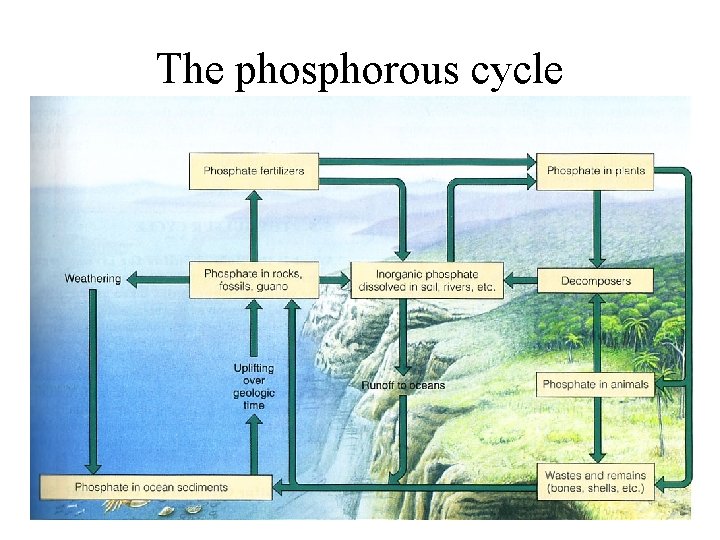
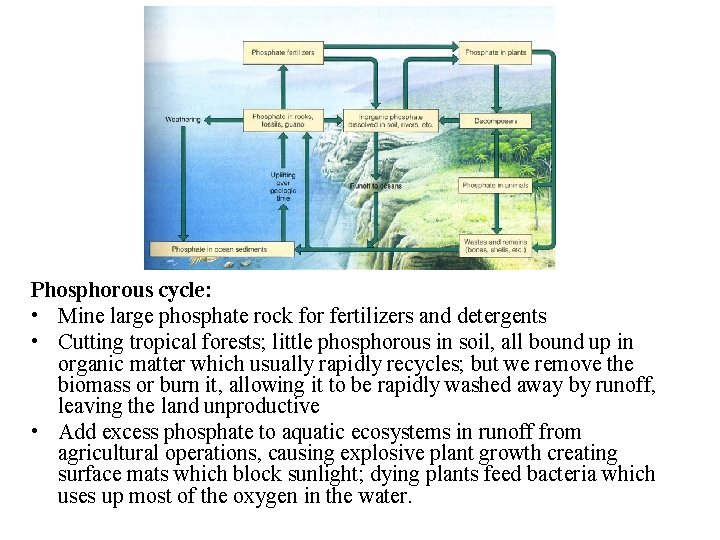
The phosphorous cycle

• essential nutrient of plants and animals, used in DNA, nucleic acids, fats, cell membranes, and bones, teeth and shells • from phosphate deposits on land shallow ocean sediments to living organisms and slowly back to the land ocean • Very little in the atmosphere, only as small particles of dust • much more rapidly through living components than through geological formations; animals get by eating producers or animals that eat producers • Animal wastes and decay return much of this phosphorous to the soil, streams, and eventually to ocean bottom and into rock cycle • Hydrologic, atmospheric or sedimentary?

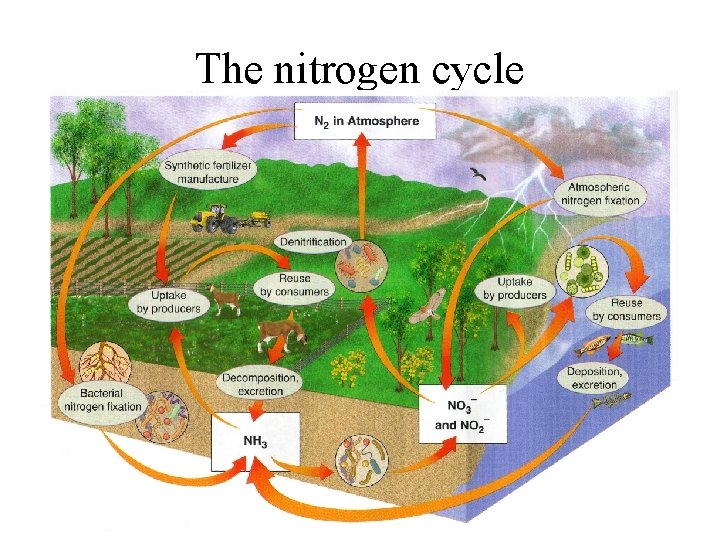
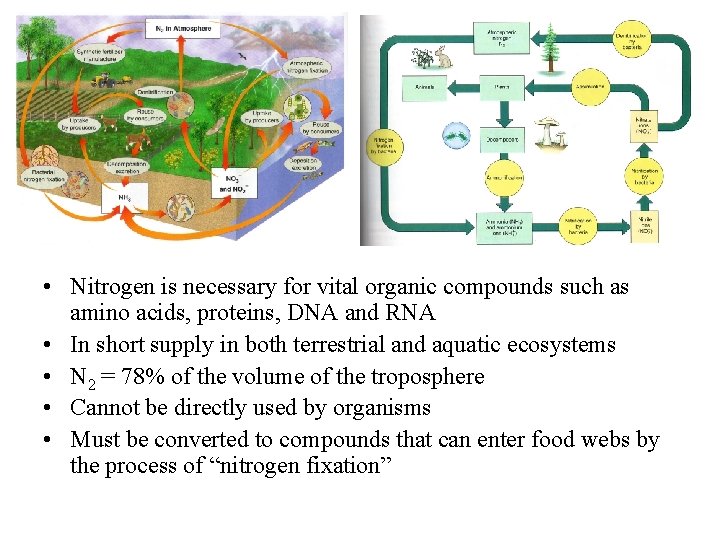
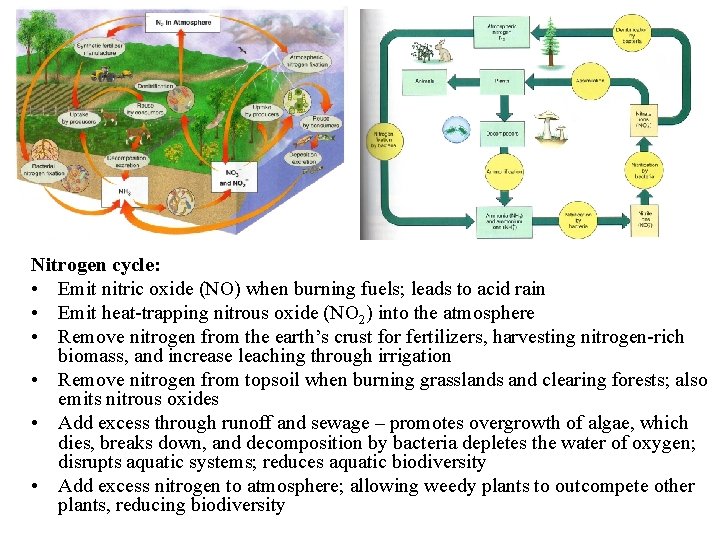
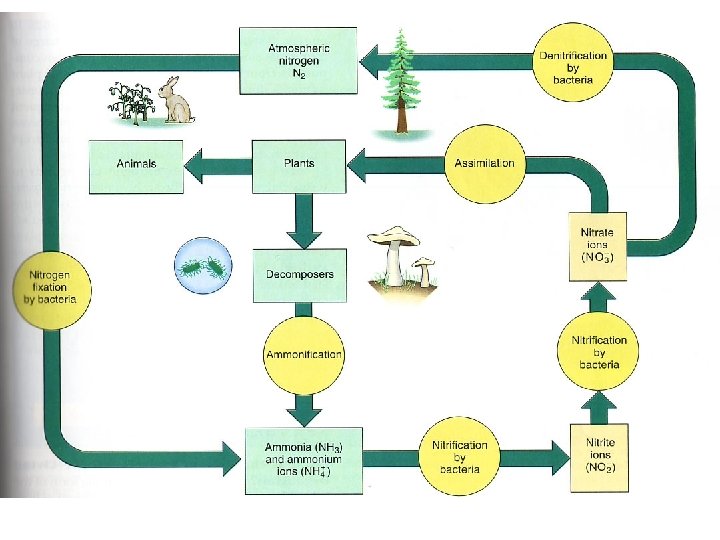
The nitrogen cycle

• Nitrogen is necessary for vital organic compounds such as amino acids, proteins, DNA and RNA • In short supply in both terrestrial and aquatic ecosystems • N 2 = 78% of the volume of the troposphere • Cannot be directly used by organisms • Must be converted to compounds that can enter food webs by the process of “nitrogen fixation”

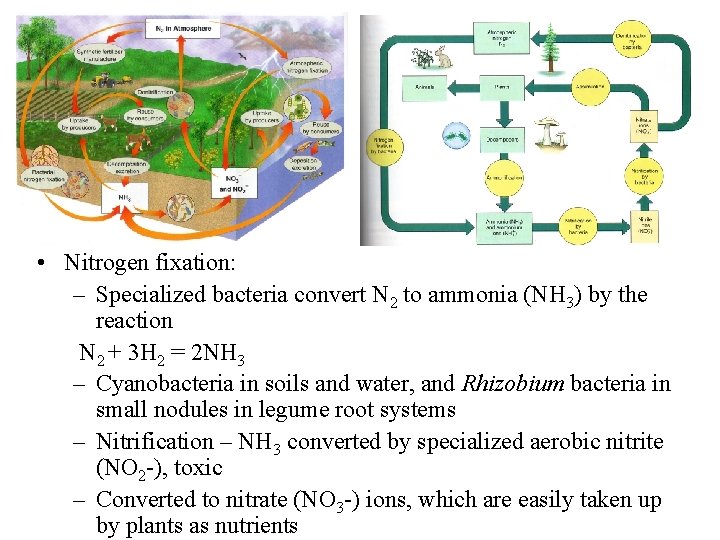
• Nitrogen fixation: – Specialized bacteria convert N 2 to ammonia (NH 3) by the reaction N 2 + 3 H 2 = 2 NH 3 – Cyanobacteria in soils and water, and Rhizobium bacteria in small nodules in legume root systems – Nitrification – NH 3 converted by specialized aerobic nitrite (NO 2 -), toxic – Converted to nitrate (NO 3 -) ions, which are easily taken up by plants as nutrients

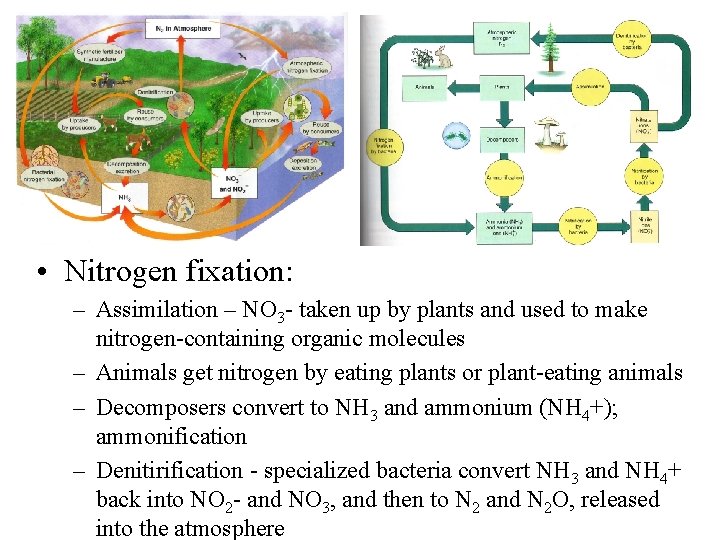
• Nitrogen fixation: – Assimilation – NO 3 - taken up by plants and used to make nitrogen-containing organic molecules – Animals get nitrogen by eating plants or plant-eating animals – Decomposers convert to NH 3 and ammonium (NH 4+); ammonification – Denitirification - specialized bacteria convert NH 3 and NH 4+ back into NO 2 - and NO 3, and then to N 2 and N 2 O, released into the atmosphere

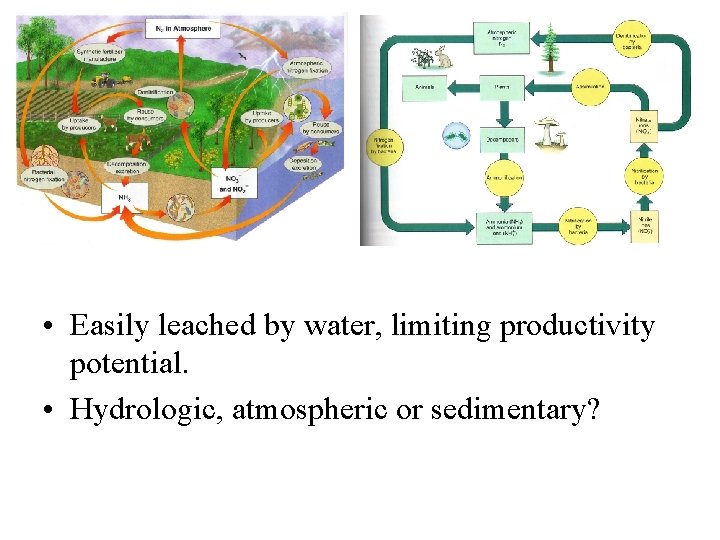
• Easily leached by water, limiting productivity potential. • Hydrologic, atmospheric or sedimentary?

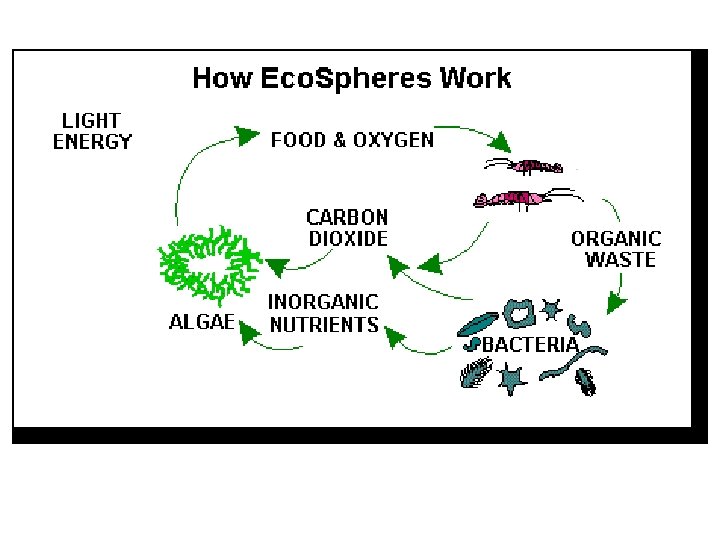
Back to the Ecosphere


How do humans affect nutrient cycles?

Water cycle: • Drain fresh water from streams, lakes, and underground sources • Clear vegetation increasing runoff, reducing infiltration, increasing erosion and risk of flooding • Modify water quality by adding nutrients (phosphates) and changing ecological processes that naturally purify water

Carbon cycle: • Put more CO 2 in the atmosphere than plants can remove • Deforestation reduces the amount of vegetation to remove CO 2 • Burning fossil fuels and wood releases more CO 2 than natural processes • What happens when we have to much heat-trapping gas?

Phosphorous cycle: • Mine large phosphate rock for fertilizers and detergents • Cutting tropical forests; little phosphorous in soil, all bound up in organic matter which usually rapidly recycles; but we remove the biomass or burn it, allowing it to be rapidly washed away by runoff, leaving the land unproductive • Add excess phosphate to aquatic ecosystems in runoff from agricultural operations, causing explosive plant growth creating surface mats which block sunlight; dying plants feed bacteria which uses up most of the oxygen in the water.

Nitrogen cycle: • Emit nitric oxide (NO) when burning fuels; leads to acid rain • Emit heat-trapping nitrous oxide (NO 2) into the atmosphere • Remove nitrogen from the earth’s crust for fertilizers, harvesting nitrogen-rich biomass, and increase leaching through irrigation • Remove nitrogen from topsoil when burning grasslands and clearing forests; also emits nitrous oxides • Add excess through runoff and sewage – promotes overgrowth of algae, which dies, breaks down, and decomposition by bacteria depletes the water of oxygen; disrupts aquatic systems; reduces aquatic biodiversity • Add excess nitrogen to atmosphere; allowing weedy plants to outcompete other plants, reducing biodiversity

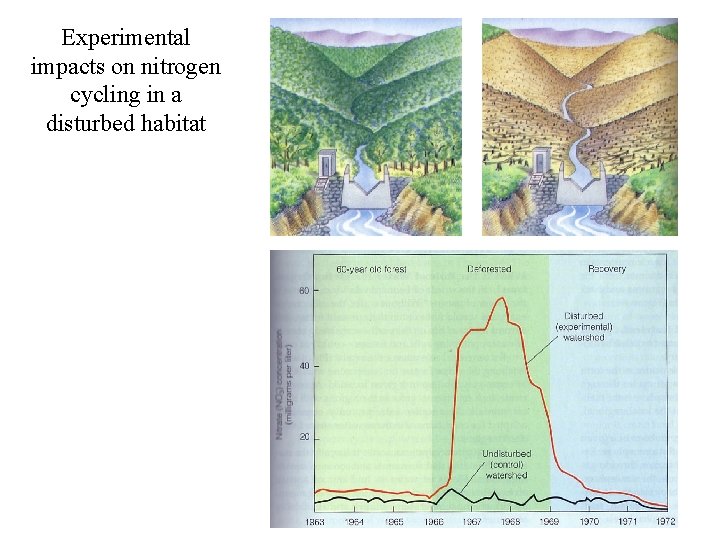
Experimental impacts on nitrogen cycling in a disturbed habitat

Nitrogen cycles in an experimental ecosystem

 Culture medium
Culture medium Ganesha ecosphere
Ganesha ecosphere Hsrproj
Hsrproj Ecosphere
Ecosphere Apes cycles
Apes cycles Nutrient cycles
Nutrient cycles A terrestrial food web
A terrestrial food web Immobile nutrients
Immobile nutrients Nutrient agar with tellurite is selective/differential for
Nutrient agar with tellurite is selective/differential for What is fat made of
What is fat made of Which ecosystem is this nutrient cycle from?
Which ecosystem is this nutrient cycle from? Bray nutrient mobility concept
Bray nutrient mobility concept Foods used in nutrition activities should be nutrient-dense
Foods used in nutrition activities should be nutrient-dense Interfering agent eggs
Interfering agent eggs Tamoxifen nutrient depletion
Tamoxifen nutrient depletion Deep venous palmar arch
Deep venous palmar arch Nutrient basics
Nutrient basics Recipes that use eggs as an emulsifier
Recipes that use eggs as an emulsifier 4 types of salad
4 types of salad Defination of nutrient
Defination of nutrient Energy in trophic levels
Energy in trophic levels How does energy flow in an ecosystem
How does energy flow in an ecosystem Nutrient cycle of a tropical rainforest
Nutrient cycle of a tropical rainforest Nutrient agar plate
Nutrient agar plate Nutrient deficiency in tomatoes
Nutrient deficiency in tomatoes Classification of nutrients and their functions
Classification of nutrients and their functions Nutrient cycle in the serengeti
Nutrient cycle in the serengeti Integrated nutrient management for sustainable agriculture
Integrated nutrient management for sustainable agriculture