Nutrient Cycles The Water Cycle 1 Water cycles










- Slides: 17

Nutrient Cycles

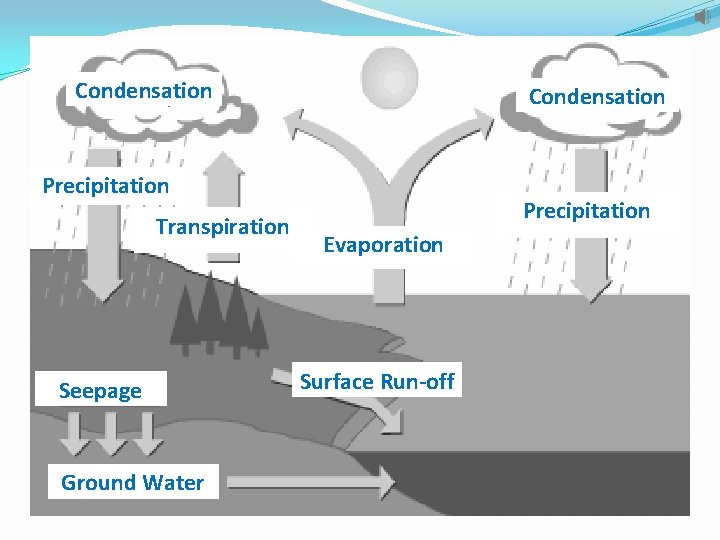
The Water Cycle 1. Water cycles between the oceans, atmosphere and land. All living organisms require water. Water enters the atmosphere as water vapor, a gas, when water evaporates from the ocean or other bodies of water. Evaporation—the process by which water changes from a liquid to a gas. Water can also enter the atmosphere by evaporating from the leaves of plants—Transpiration. a. The sun heats the atmosphere. b. Warm, moist air rises and cools.

Water Cycle Condensation-- Eventually, the water vapor condenses into tiny droplets that form clouds. d. When the droplets become large enough, the water return to Earth’s surface. Precipitation--rain, snow, sleet, or hail

Water Cycle D. Run-off—Precipitation runs along the surface of the ground until it enters a river or a stream that carries the run-off back to an ocean or lake. E. Seepage—Rain also seeps into the soil, some of it deeply enough to become ground water. Water in the soil enters plants through the roots, and the water cycle begins anew.

Condensation Precipitation Transpiration Seepage Ground Water Precipitation Evaporation Surface Run-off

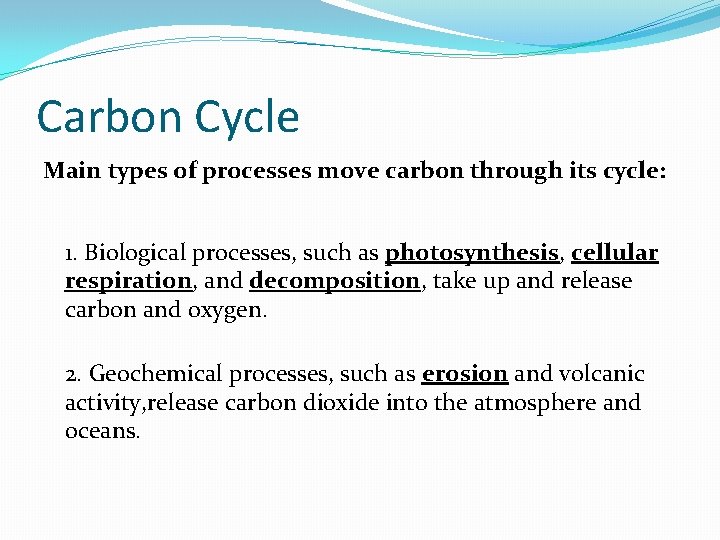
The Carbon Cycle 1. Every organic molecule contains the element carbon. Carbon and oxygen form carbon dioxide gas (CO 2), an important component of the atmosphere. Carbon dioxide is taken in by plants during photosynthesis and is given off by plants and animals during cellular respiration.

Carbon Cycle Main types of processes move carbon through its cycle: 1. Biological processes, such as photosynthesis, cellular respiration, and decomposition, take up and release carbon and oxygen. 2. Geochemical processes, such as erosion and volcanic activity, release carbon dioxide into the atmosphere and oceans.

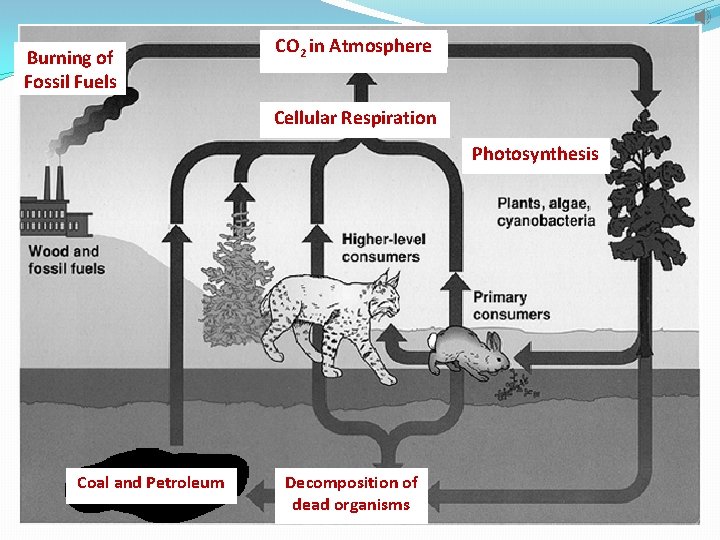
Carbon Cycle 3. Mixed biogeochemical processes, such as the burial and decomposition of dead organisms and their conversion under pressure into coal and petroleum (fossil fuels), store carbon underground. 4. Human activities, such as mining, cutting and burning forests, and burning fossil fuels, release carbon dioxide into the atmosphere.

Burning of Fossil Fuels CO 2 in Atmosphere Cellular Respiration Photosynthesis Coal and Petroleum Decomposition of dead organisms

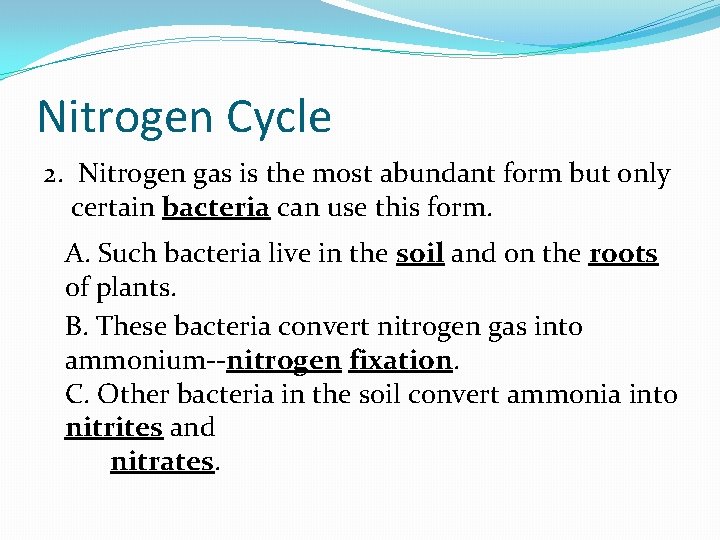
The Nitrogen Cycle 1. All organisms require nitrogen to make amino acids, which in turn are used to build proteins. A. Nitrogen gas makes up 78% of Earth’s atmosphere. B. Nitrogen containing substances such as ammonia (NH 3), nitrites (NO 2 -), and nitrates (NO 3 -) are found in the wastes produced by many organisms and in dead and decaying organic matter. C. Nitrate is major component of plant fertilizers.

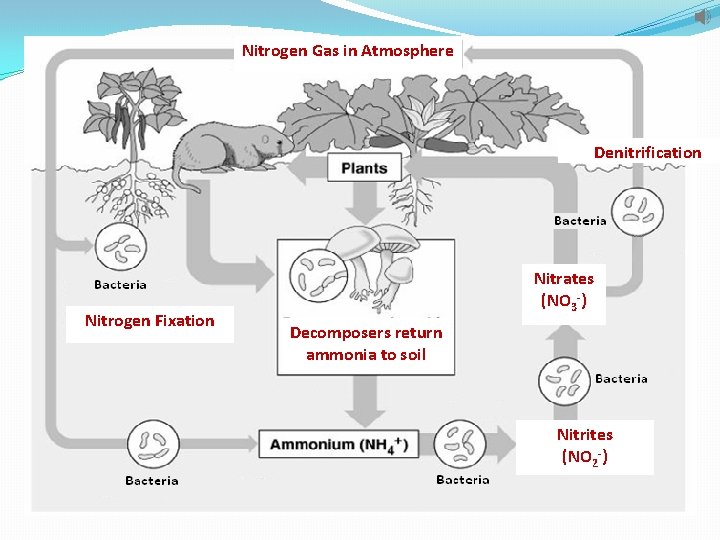
Nitrogen Cycle 2. Nitrogen gas is the most abundant form but only certain bacteria can use this form. A. Such bacteria live in the soil and on the roots of plants. B. These bacteria convert nitrogen gas into ammonium--nitrogen fixation. C. Other bacteria in the soil convert ammonia into nitrites and nitrates.

Nitrogen Cycle 3. Once the nitrites and nitrates are available, producers (plants) can use them to make proteins. Consumers then eat the producers and reuse the nitrogen to make their own proteins. 4. When organisms die, decomposers return nitrogen to the soil as ammonia. 5. Other soil bacteria convert nitrates into nitrogen gas-denitrification. This process releases nitrogen into the atmosphere once again.

Nitrogen Gas in Atmosphere Denitrification Nitrogen Fixation Nitrates (NO 3 -) Decomposers return ammonia to soil Nitrites (NO 2 -)

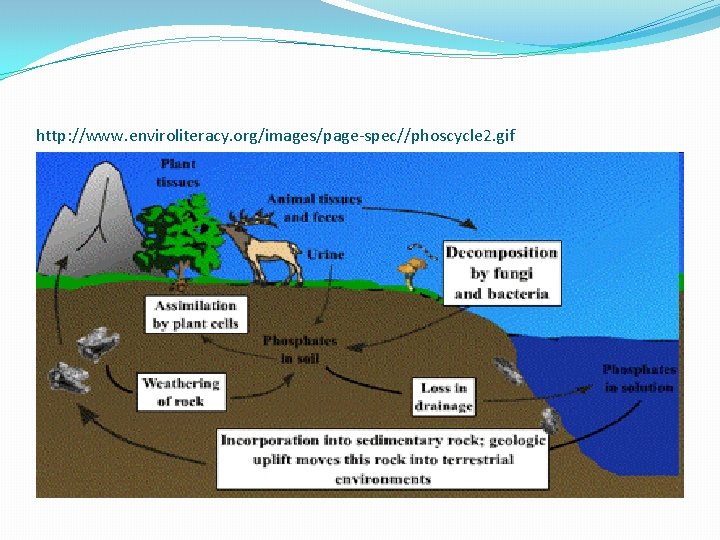
Phosphorus �Phosphorus is necessary for plants and animals. �Phosphorus is the building block of important parts of the body such as bones and teeth. �Phosphorus does not enter the atmosphere--it stays in rocks and soil minerals.

Phosphorus �Unlike other cycles of matter compounds, phosphorus cannot be found in atmosphere as a gas. �It usually cycles through water, soil, and sediments.

Phosphorous Cycle �First phosphorus is eroded off of rocks and deposited in the soil. �Once in the soil, phosphorus is absorbed into the clay and other organic material within the soil. �Next plants dissolve the phosphates in the soil. �Then the phosphates are absorbed by herbivores when they consume the plants and then into carnivores when they eat the herbivores. �Finally phosphorus is deposited back into the soil when plants and animals decay or through animal waste.

http: //www. enviroliteracy. org/images/page-spec//phoscycle 2. gif