Nutrient Cycles in Marine Ecosystems Part I Section





- Slides: 13

Nutrient Cycles in Marine Ecosystems Part I Section 4

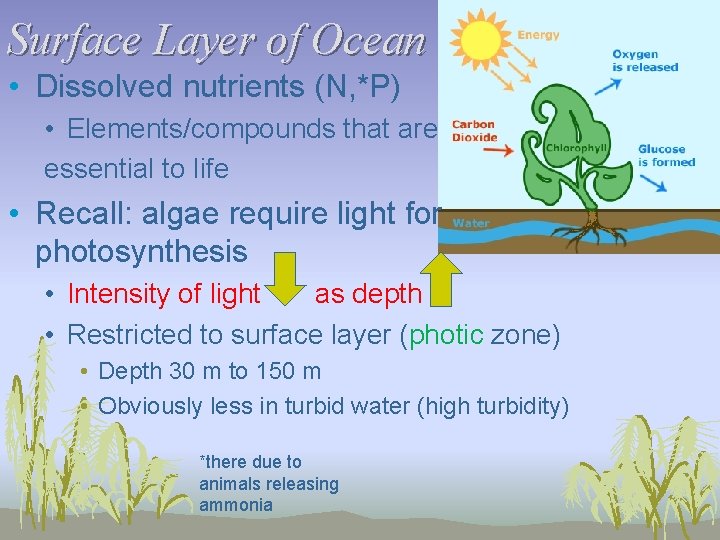
Surface Layer of Ocean • Dissolved nutrients (N, *P) • Elements/compounds that are essential to life • Recall: algae require light for photosynthesis • Intensity of light as depth • Restricted to surface layer (photic zone) • Depth 30 m to 150 m • Obviously less in turbid water (high turbidity) *there due to animals releasing ammonia

What is an ion? • atom or molecule in which the total number of electrons is not equal to the total number of protons, giving the atom a net positive (+) or negative (-) electrical charge.

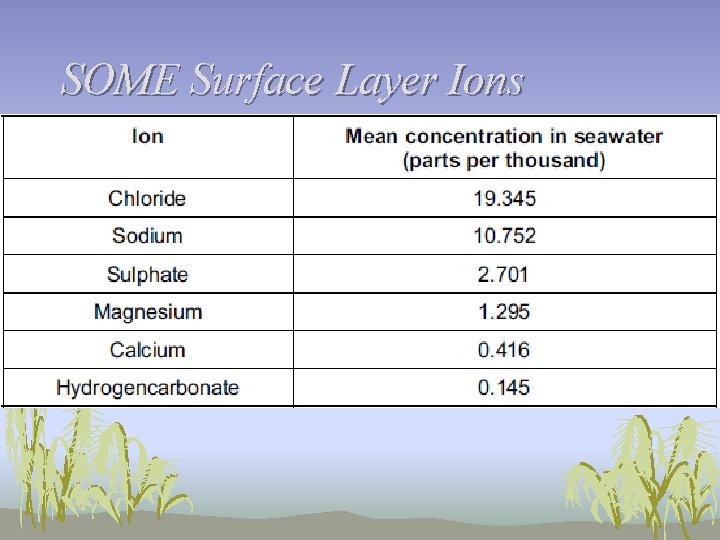
SOME Surface Layer Ions

Surface Ions • Those ions, together with nitrate and phosphate ions form nutrients for growth of algae and other producers • Unit of measurement: ppm • Nitrate and phosphate ions occur in low concentrations in seawater • Nitrate = 0. 5 ppm • Phosphate = 0. 07 ppm

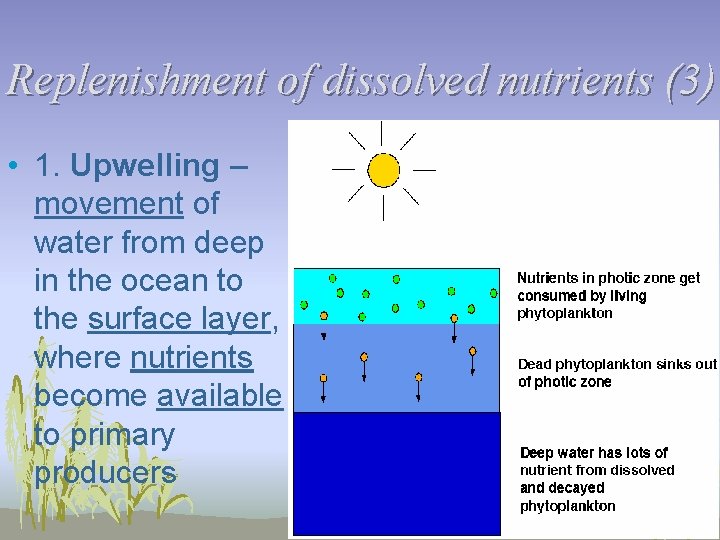
Replenishment of dissolved nutrients (3) • 1. Upwelling – movement of water from deep in the ocean to the surface layer, where nutrients become available to primary producers

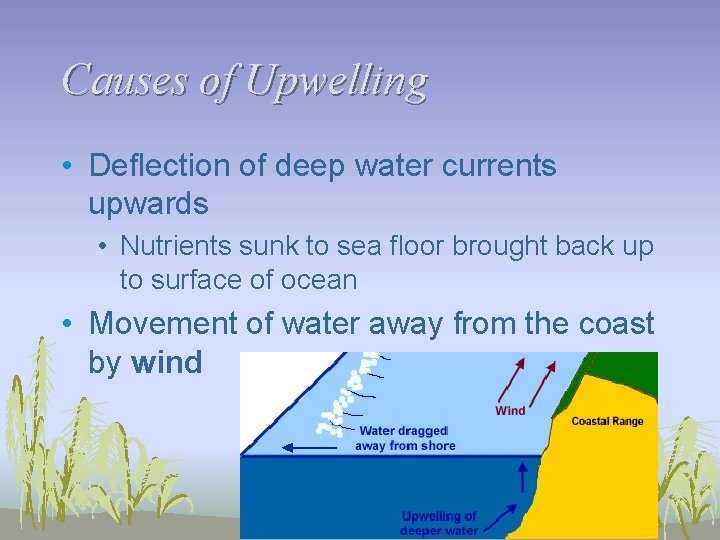
Causes of Upwelling • Deflection of deep water currents upwards • Nutrients sunk to sea floor brought back up to surface of ocean • Movement of water away from the coast by wind

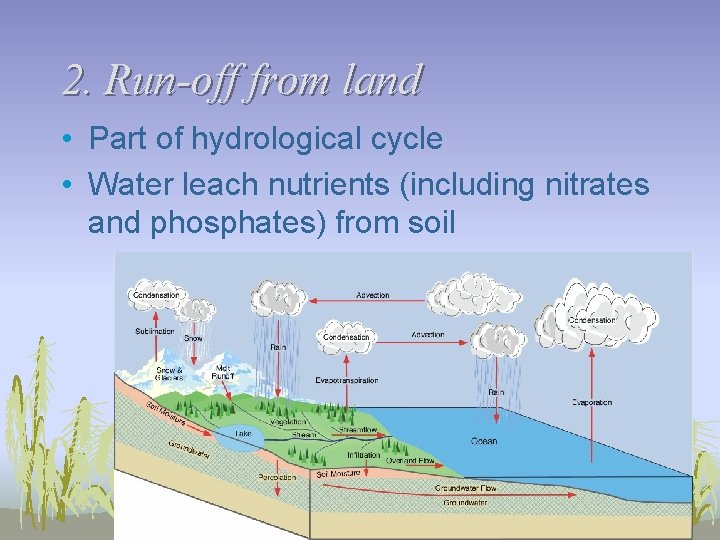
2. Run-off from land • Part of hydrological cycle • Water leach nutrients (including nitrates and phosphates) from soil

3. Atmospheric Gases • CO 2 dissolved in seawater forming hydrogencarbonate ions (HCO 3 -) • AKA bicarbonate • Makes CO 2 available for fixation in photosynthesis for primary producers • *Ocean acidification • N gas is fixed by blue-green bacteria in intertidal zones • Forms nitrogen-containing organic compounds • How nitrogen enters marine ecosystems

Intertidal Zone Area above water during low tide but underwater during high tide

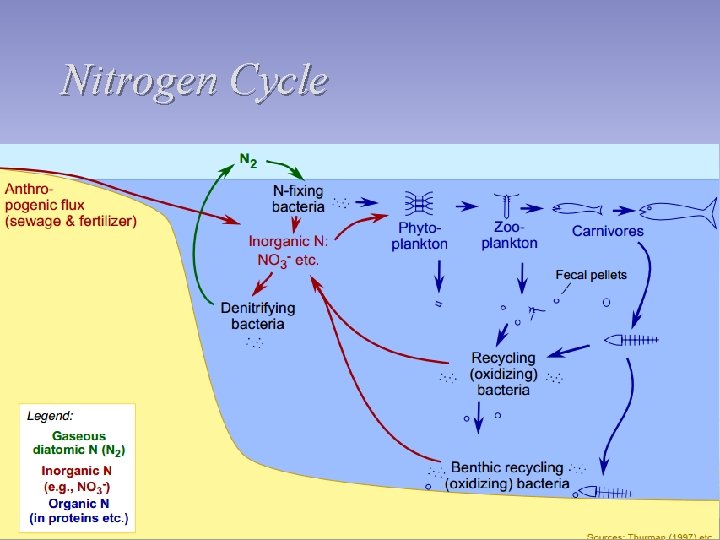
Nitrogen Cycle

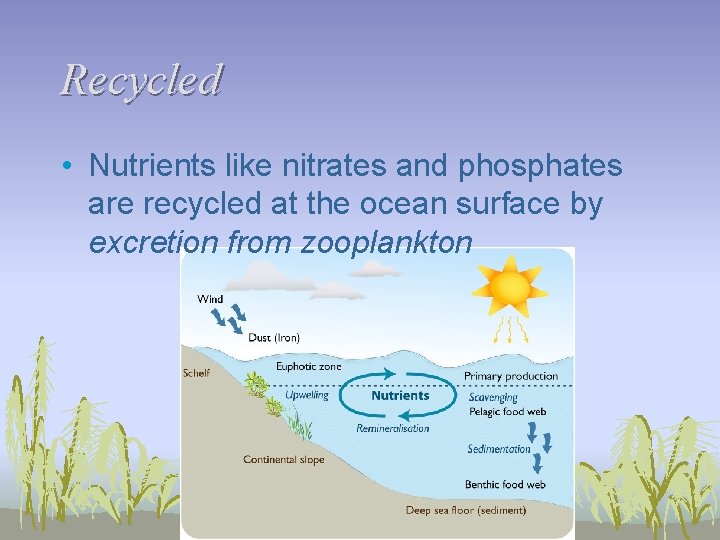
Recycled • Nutrients like nitrates and phosphates are recycled at the ocean surface by excretion from zooplankton

Depletion of dissolved nutrients • Uptake by primary producers like phytoplankton • Synthesis of organic substances • Example: nitrate ions used for synthesis of amino acids and proteins • Phytoplankton zooplankton small fish • Proteins passed to next trophic level and so forth