Introduction to the Control of Listeria monocytogenes Lm















- Slides: 19

Introduction to the Control of Listeria monocytogenes (Lm) in Ready-to-Eat Products; Interim Final Rule Small and Very Small Establishment Implementation Workshop 1

Control of Lm in RTE products Ø Background l FMIA, PPIA, EPIA • wholesome, not adulterated, and properly marked, labeled, and packaged. l FMIA and PPIA: Adulteration • bears or contains any poisonous or deleterious substance that may render it injurious to health • been prepared, packed, or held under insanitary conditions 2

Control of Lm in RTE products Ø Background: l l l During the 1980’s, Lm began to emerge as a problem in processed meat and poultry products. In the 1990’s, outbreaks of foodborne illness caused by Lm. From 1999 -2003 various Agency publications were issued addressing Lm 3

Control of Lm in RTE products Ø Background: l Federal Register Interim Final Rule 6/6/2003 • Control of Listeria monocytogenes in RTE Meat and Poultry Products; Final Rule • 9 CFR Part 430 4

Control of Lm in RTE products Ø Implementation of new RTE regulations l l l Why do I need to make changes? How does this affect establishment’s producing RTE products? What are the changes or new requirements? When will I be required to make the change? Will I need to modify my SSOP and/or HACCP plan? 5

§ 430. 4 Control of Lm in Postlethality Exposed RTE Products Lm can contaminate RTE products that are exposed to the environment after a lethality treatment (destroy/kill). Ø Lm is a hazard that an establishment must control through its HACCP plan, or prevent in the environment through a SSOP or other prerequisite program if it produces RTE product that is exposed post-lethality. Ø RTE product is adulterated if it contains Lm or if it contacts surfaces contaminated with Lm. Ø 6

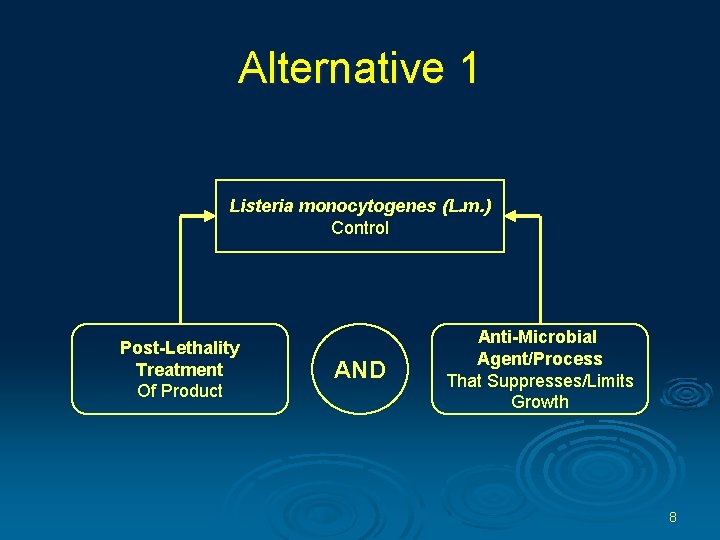
Control of Lm in Post-lethality Exposed RTE Products Ø In order to maintain sanitary conditions necessary to meet this requirement, an establishment producing post-lethality exposed RTE product must comply with one of three alternatives. 7

Alternative 1 Listeria monocytogenes (L. m. ) Control Post-Lethality Treatment Of Product AND Anti-Microbial Agent/Process That Suppresses/Limits Growth 8

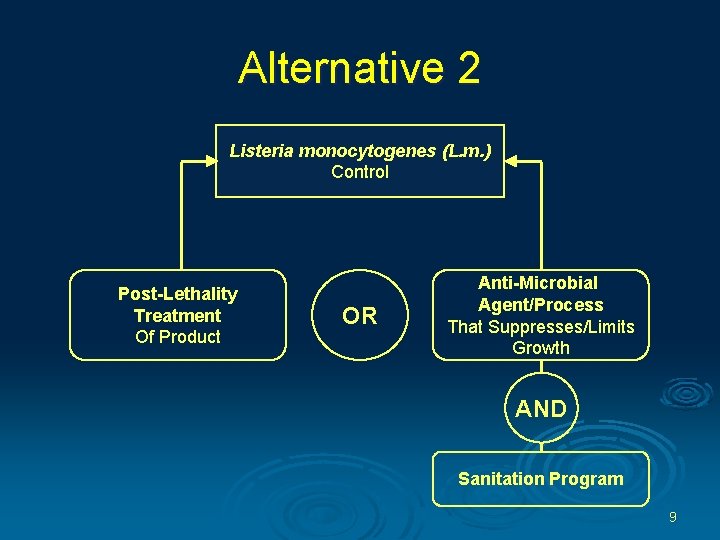
Alternative 2 Listeria monocytogenes (L. m. ) Control Post-Lethality Treatment Of Product OR Anti-Microbial Agent/Process That Suppresses/Limits Growth AND Sanitation Program 9

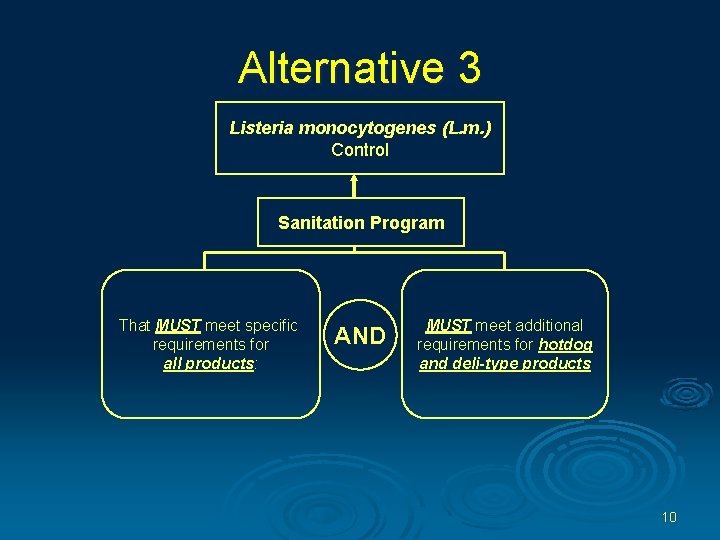
Alternative 3 Listeria monocytogenes (L. m. ) Control Sanitation Program That MUST meet specific requirements for all products: AND MUST meet additional requirements for hotdog and deli-type products 10

Risk to Product Risk Alternative 3 > Alternative 2 > Alternative 1 11

For All Three Alternatives 1) Establishments may use verification testing, which would be in addition to FSIS verification testing, that includes tests for Lm or an indicator organism, such as Listeria species, to verify the effectiveness of their sanitation procedures in the post-lethality processing environment. 12

For All Three Alternatives (cont. ) 2) Sanitation measures and procedures for antimicrobial agents or processes that control Lm may be incorporated either in the establishment’s HACCP plan or in its SSOP or other prerequisite programs. If these control procedures are included in the SSOP or prerequisite program, and not as a CCP in the HACCP plan, the establishment must have documentation supporting the decision in its hazard analysis that Lm is not a hazard reasonably likely to occur. 13

For All Three Alternatives (cont. ) Establishments must maintain sanitation in the post-lethality environment in accordance with part 416. 4) If Lm control measures are included in the HACCP plan, the establishment must validate and verify the effectiveness of these Lm control measures in accordance with § 417. 4. 3) 14

For All Three Alternatives (cont. ) If Lm control measures are included in the SSOP, the effectiveness of these measures must be evaluated in accordance with § 416. 14. 6) If the Lm control measures are included in a prerequisite program other than the SSOP, the program and the results produced by the program must be included in the documentation that establishment is required to maintain in accordance with § 417. 5. 5) 15

For All Three Alternatives (cont. ) 7) The establishment must make the verification results that demonstrate the effectiveness of the Lm control measures it employs, whether under its HACCP plan or SSOP or other prerequisite program(s), available to FSIS inspection personnel upon request. 16

Supplying Information to FSIS An establishment that produced postlethality exposed RTE product shall provide FSIS, at least annually, or more often as determined by the Administrator, with estimates of annual production volume and related information for the types of meat and poultry products processed under each alternative specified in § 430. 4(b). 17

Labeling Establishments that control Lm by using a post-lethality treatment or an antimicrobial agent or process that eliminates or reduces, or suppresses or limits the growth of Lm, may declare this fact on the product label provided that the establishment has validated the claim. 18

Workshop Breakout sessions 19
 Listeria
Listeria Reverse camp test listeria
Reverse camp test listeria Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Lp html
Lp html Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Chụp tư thế worms-breton
Chụp tư thế worms-breton Chúa yêu trần thế
Chúa yêu trần thế Các môn thể thao bắt đầu bằng tiếng nhảy
Các môn thể thao bắt đầu bằng tiếng nhảy Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tính thế năng
Công thức tính thế năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Cách giải mật thư tọa độ
Cách giải mật thư tọa độ Phép trừ bù
Phép trừ bù độ dài liên kết
độ dài liên kết Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra