Introduction to Atoms Ions and Isotopes Honors Coordinated







- Slides: 15

Introduction to Atoms, Ions and Isotopes Honors Coordinated Science II Wheatley-Heckman

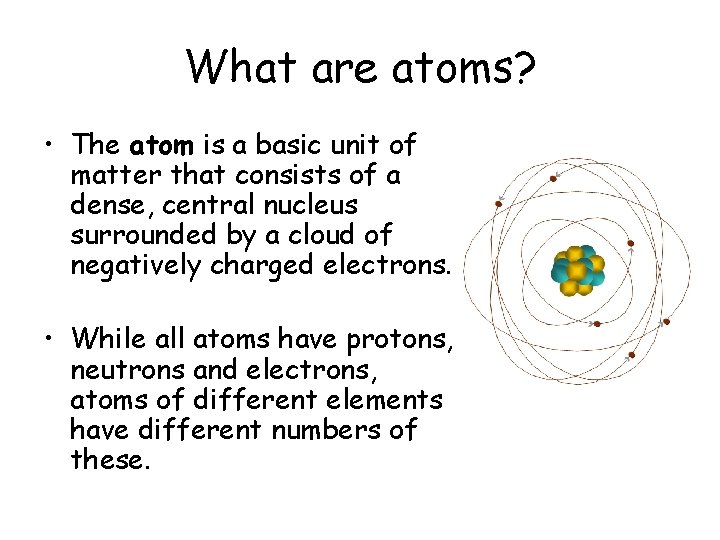
What are atoms? • The atom is a basic unit of matter that consists of a dense, central nucleus surrounded by a cloud of negatively charged electrons. • While all atoms have protons, neutrons and electrons, atoms of different elements have different numbers of these.

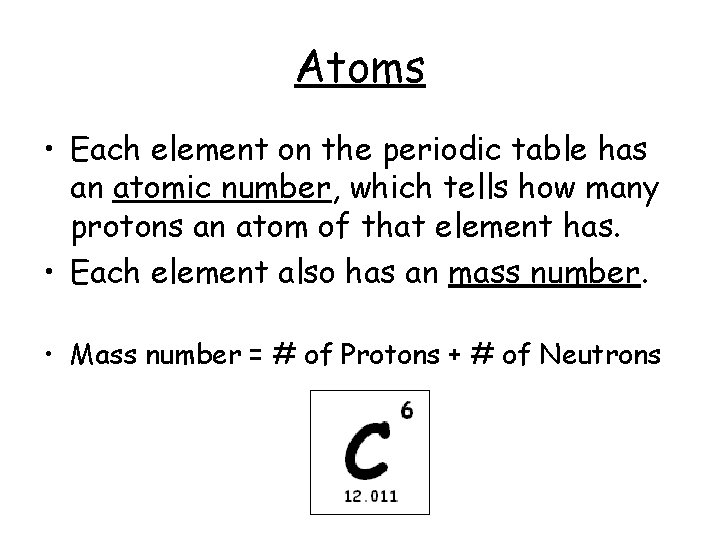
Atoms • Each element on the periodic table has an atomic number, which tells how many protons an atom of that element has. • Each element also has an mass number. • Mass number = # of Protons + # of Neutrons

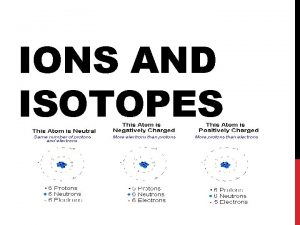
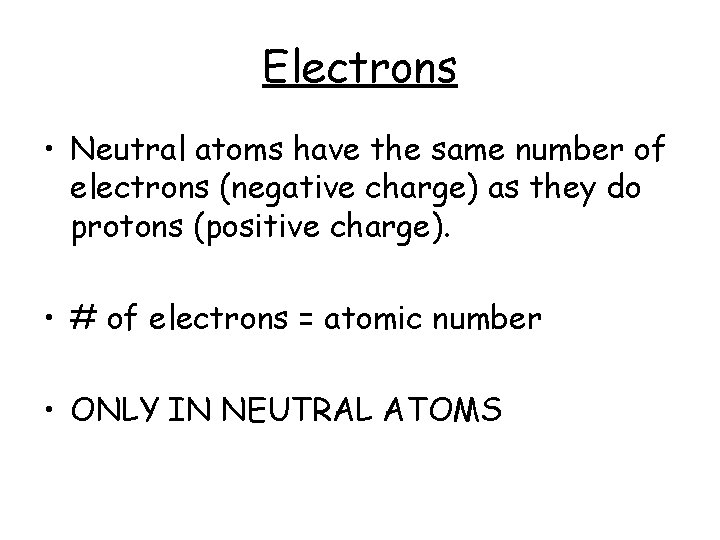
Electrons • Neutral atoms have the same number of electrons (negative charge) as they do protons (positive charge). • # of electrons = atomic number • ONLY IN NEUTRAL ATOMS

Let’s Practice! • Find Boron on the periodic table. • How many protons does an atom of Boron have? • How many neutrons? – Hint: If the mass number is a decimal, round it to the nearest whole number to calculate # of neutrons. • How many electrons?

Ions • An atom or small molecule with an overall positive or negative charge due to an imbalance of protons and electrons. – # of protons does not change – # of electrons changes. – Positive charge = loss of electrons – Negative charge = gain of electron

Ions Continued… • Ions are represented by a superscript charge on the element symbol. – Examples: F-, Mg 2+, Al 3+ Practice: • How many protons, neutrons, and electrons does Al 3+ have?

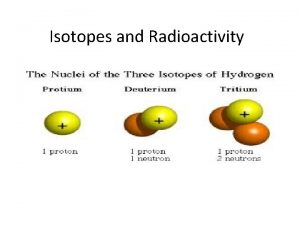
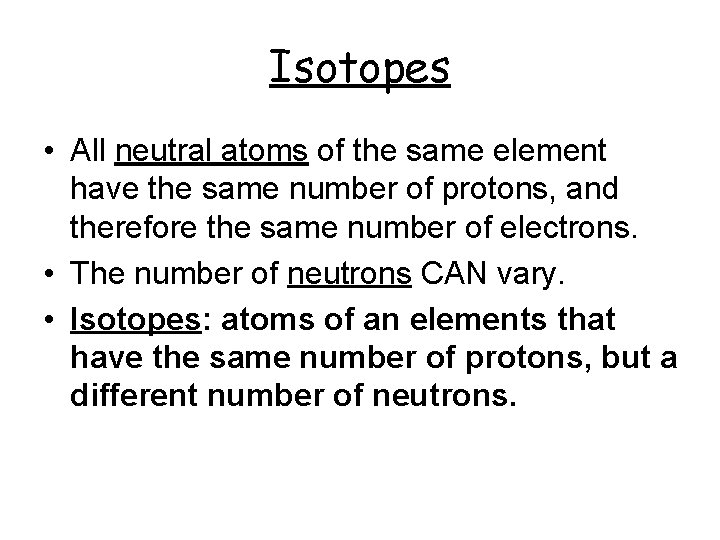
Isotopes • All neutral atoms of the same element have the same number of protons, and therefore the same number of electrons. • The number of neutrons CAN vary. • Isotopes: atoms of an elements that have the same number of protons, but a different number of neutrons.


Average Atomic Mass • The mass shown on your periodic table as a decimal is the average atomic mass. – Average mass of all isotopes of an atom in nature.

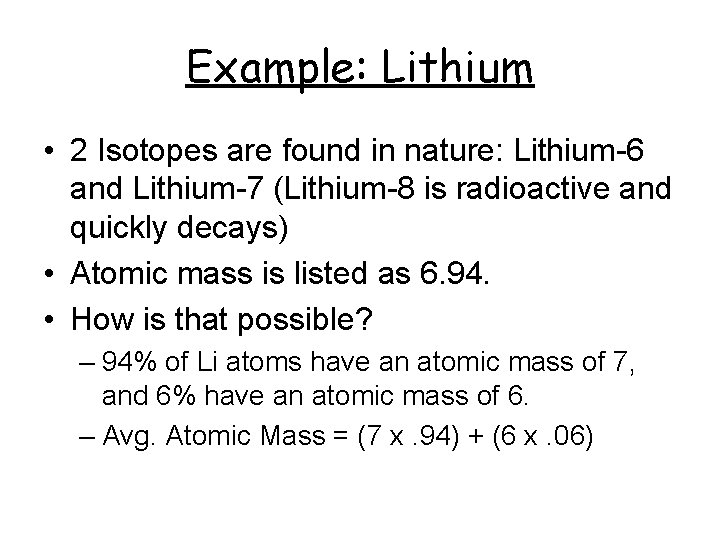
Example: Lithium • 2 Isotopes are found in nature: Lithium-6 and Lithium-7 (Lithium-8 is radioactive and quickly decays) • Atomic mass is listed as 6. 94. • How is that possible? – 94% of Li atoms have an atomic mass of 7, and 6% have an atomic mass of 6. – Avg. Atomic Mass = (7 x. 94) + (6 x. 06)

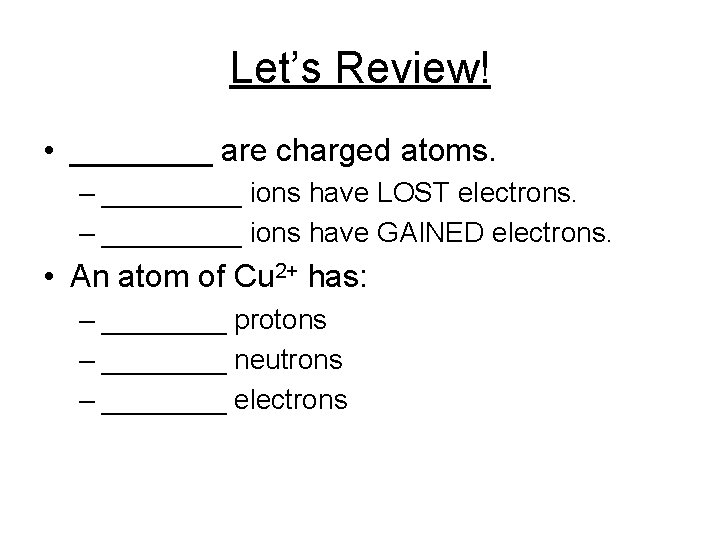
Let’s Review! • ____ are charged atoms. – _____ ions have LOST electrons. – _____ ions have GAINED electrons. • An atom of Cu 2+ has: – ____ protons – ____ neutrons – ____ electrons

More Review • ______ are atoms of the same element that have different numbers of neutrons in the nucleus. • What does average atomic mass represent?

Complete Atomic Structure WS #1 Front and Back Hint: Chromium-52 means Chromium with a mass number of 52. 26 Fe means iron with atomic number 26.

Book Work • Read section 5. 1 • Pg. 134 -143 • Answer questions 1 -3, 6, 7, 23, 29 -32, 38, 65, 71 -76.
 Atoms and their isotopes pogil
Atoms and their isotopes pogil What do the roman numerals in a cation's name indicate
What do the roman numerals in a cation's name indicate Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms molecules and ions
Atoms molecules and ions Atoms ions and molecules
Atoms ions and molecules Atoms ions and molecules
Atoms ions and molecules States that atoms ions and molecules must collide to react
States that atoms ions and molecules must collide to react Chapter 2 atoms molecules and ions
Chapter 2 atoms molecules and ions How does a positive ion form
How does a positive ion form Atoms or ions are considered isoelectronic if
Atoms or ions are considered isoelectronic if Periodic table of elements regents
Periodic table of elements regents Isotopes stable and unstable
Isotopes stable and unstable Fertile isotopes
Fertile isotopes Percentage abundance
Percentage abundance