International Symposium on the Centenary of Chagas disease







- Slides: 22

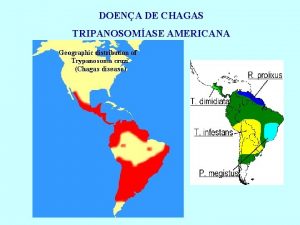
International Symposium on the Centenary of Chagas disease Discovery Therapy, diagnosis and prognosis of chronic Chagas disease: insight gained in Argentina 9, 000 y BC 1909 discover Carlos Chagas Salvador Mazza 1920 -2009 Dx 1960 -1970 Tr Sergio Sosa-Estani, MD, Ph. D. Ce. NDIE, IECS, CONICET, Buenos Aires, Argentina

Diagnosis of infection Serological tests • Complement fixation • Indirect immunofluorescence assay • Indirect hemagglutination assay • Enzyme immune assay (Muniz & Freitas 1944) (Alvarez et al. 1968) (Cerisola et al. 1962) (Voller 1975) Transfusion 2009 QUALITY CONTROL PROGRAM

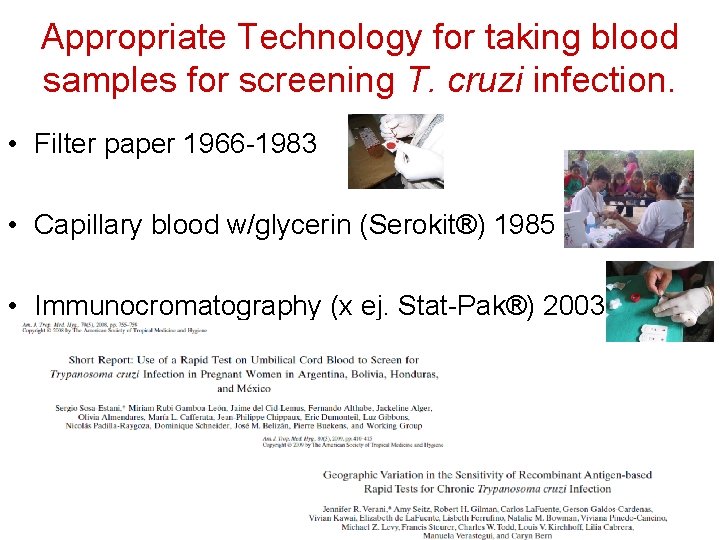
Appropriate Technology for taking blood samples for screening T. cruzi infection. • Filter paper 1966 -1983 • Capillary blood w/glycerin (Serokit®) 1985 Foto: MSF Honduras • Immunocromatography (x ej. Stat-Pak®) 2003 Foto: MS Honduras

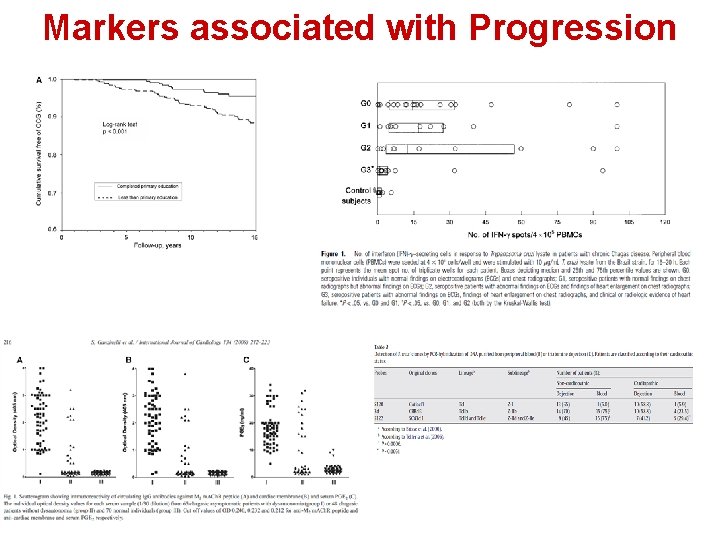
Markers associated with Progression

Prescription of Specific Treatment against T. cruzi Infection • All patients undergoing the acute phase • Children and young patients undergoing the chronic phase • Laboratory or surgical accident • Organ transplant recipients or donors • Chronic phase, indetermined or incipient cardiac form in adults may be considered, although with limited evidence

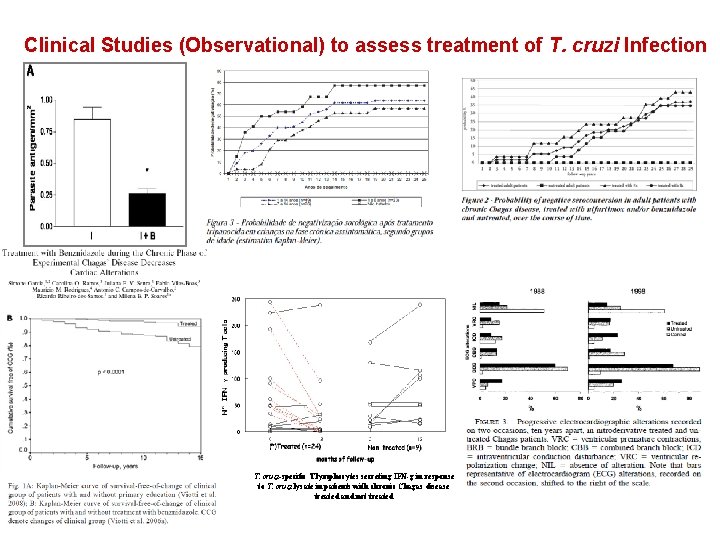
Clinical Studies (Observational) to assess treatment of T. cruzi Infection T. cruzi-specific T lymphocytes secreting IFN-g in response to T. cruzi lysate in patients with chronic Chagas disease treated and not treated

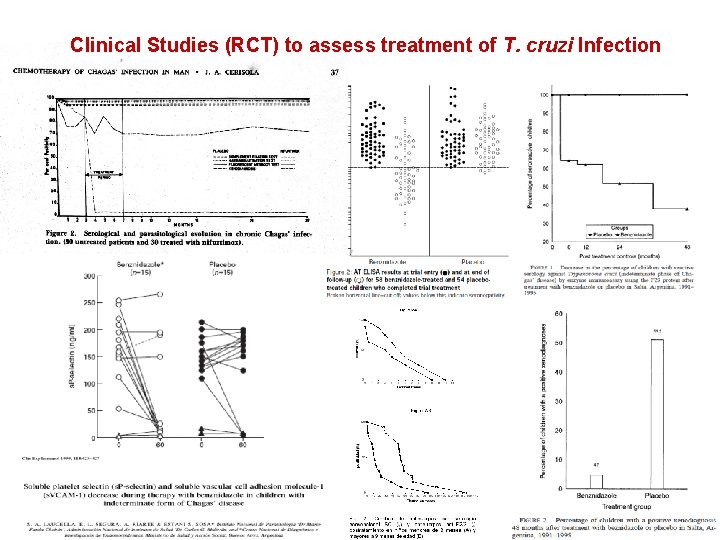
Clinical Studies (RCT) to assess treatment of T. cruzi Infection

Benznidazole Meta-analysis Villar JC, et al Cochrane Database Syst Rev. 2002; (1)

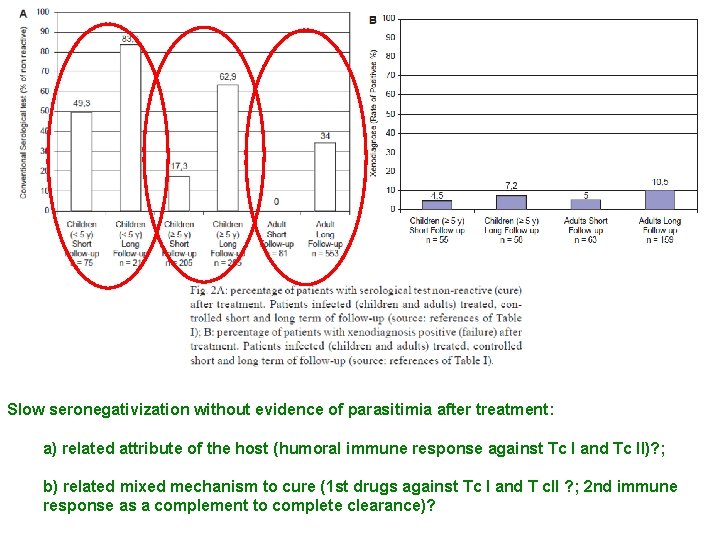
Slow seronegativization without evidence of parasitimia after treatment: a) related attribute of the host (humoral immune response against Tc I and Tc II)? ; b) related mixed mechanism to cure (1 st drugs against Tc I and T c. II ? ; 2 nd immune response as a complement to complete clearance)?

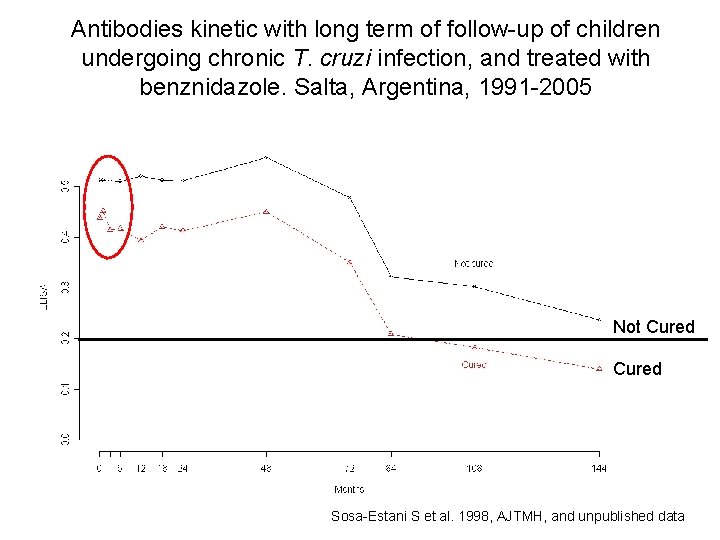
Antibodies kinetic with long term of follow-up of children undergoing chronic T. cruzi infection, and treated with benznidazole. Salta, Argentina, 1991 -2005 Not Cured Sosa-Estani S et al. 1998, AJTMH, and unpublished data

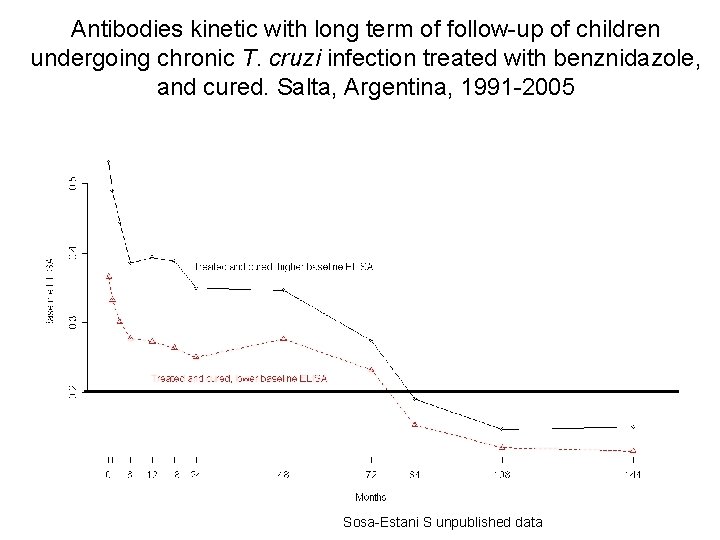
Antibodies kinetic with long term of follow-up of children undergoing chronic T. cruzi infection treated with benznidazole, and cured. Salta, Argentina, 1991 -2005 Sosa-Estani S unpublished data

Yun O et al. PLo. S Negl Trop Dis. 2009 Jul 7; 3(7): e 488.

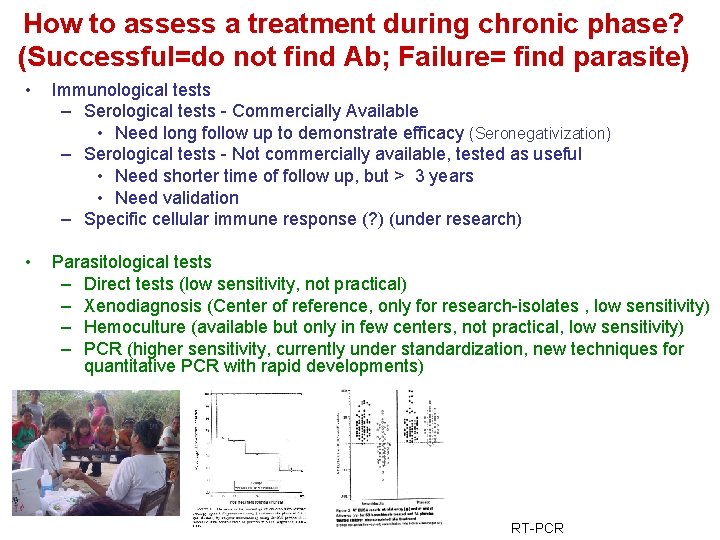
How to assess a treatment during chronic phase? (Successful=do not find Ab; Failure= find parasite) • Immunological tests – Serological tests - Commercially Available • Need long follow up to demonstrate efficacy (Seronegativization) – Serological tests - Not commercially available, tested as useful • Need shorter time of follow up, but > 3 years • Need validation – Specific cellular immune response (? ) (under research) • Parasitological tests – Direct tests (low sensitivity, not practical) – Xenodiagnosis (Center of reference, only for research-isolates , low sensitivity) – Hemoculture (available but only in few centers, not practical, low sensitivity) – PCR (higher sensitivity, currently under standardization, new techniques for quantitative PCR with rapid developments) RT-PCR

Some strategies to getting new treatments • New schemes / New prescriptions (i. e. BENEFIT Project. Efficacy of Bz in Patients with cardiac disease) • Pediatric formulation (i. e. dispersable tables and suspention-LAFEPEDNDi, Solution-UNR) • Registered drugs with anti-T. cruzi activity (i. e Posaconazole, Itraconazole (antimicotic), Bisphonates (osteoporosis) , Miltefosine (antineoplastic, antiprotozoal), Clomipramine (tricyclic antidepressant), Liposomal amphotericin (antifungical, antiprotozoal) • Evaluation of Combination (i. e. Combination of registered compounds (Benznidazole/Nifurtimox) with drugs with demonstrated activity in Chagas’ disease) • Evaluation of library of existing compounds (i. e. Furazolidone, Clemastine) • Develop an specific new drug (i. e. inhibitors of trans-sialidase, cysteine proteinase, trypanothione reductase, others)

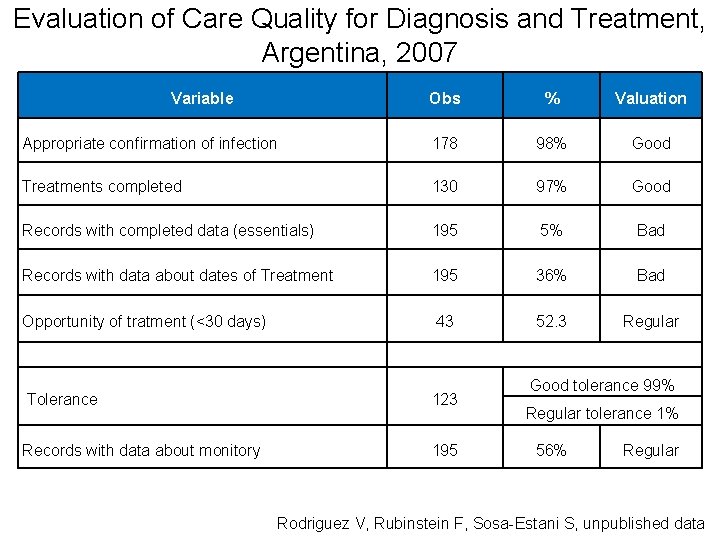
Evaluation of Care Quality for Diagnosis and Treatment, Argentina, 2007 Variable Obs % Valuation Appropriate confirmation of infection 178 98% Good Treatments completed 130 97% Good Records with completed data (essentials) 195 5% Bad Records with data about dates of Treatment 195 36% Bad Opportunity of tratment (<30 days) 43 52. 3 Regular Tolerance 123 Records with data about monitory 195 Good tolerance 99% Regular tolerance 1% 56% Regular Rodriguez V, Rubinstein F, Sosa-Estani S, unpublished data

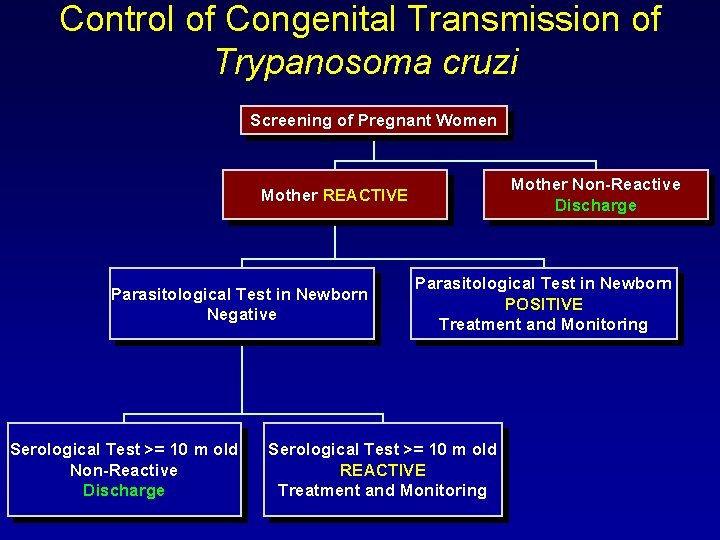
Control of Congenital Transmission of Trypanosoma cruzi Screening of Pregnant Women Mother Non-Reactive Discharge Mother REACTIVE Parasitological Test in Newborn Negative Serological Test >= 10 m old Non-Reactive Discharge Parasitological Test in Newborn POSITIVE Treatment and Monitoring Serological Test >= 10 m old REACTIVE Treatment and Monitoring

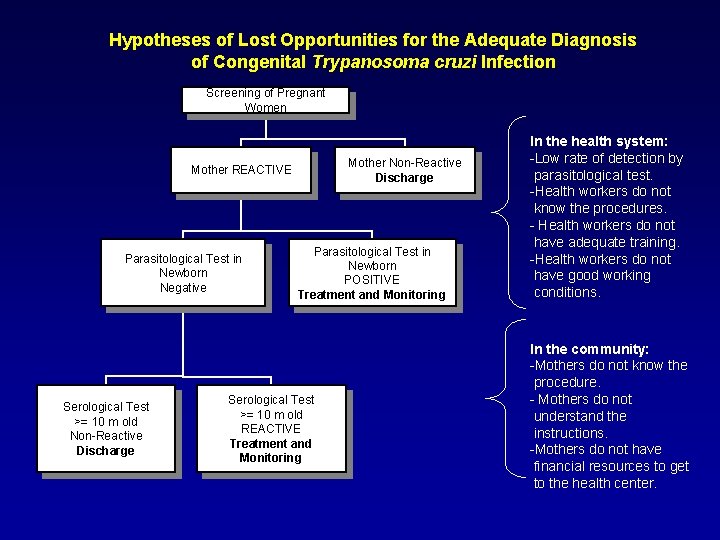
Hypotheses of Lost Opportunities for the Adequate Diagnosis of Congenital Trypanosoma cruzi Infection Screening of Pregnant Women Mother Non-Reactive Discharge Mother REACTIVE Parasitological Test in Newborn Negative Serological Test >= 10 m old Non-Reactive Discharge Parasitological Test in Newborn POSITIVE Treatment and Monitoring Serological Test >= 10 m old REACTIVE Treatment and Monitoring In the health system: -Low rate of detection by parasitological test. -Health workers do not know the procedures. - Health workers do not have adequate training. -Health workers do not have good working conditions. In the community: -Mothers do not know the procedure. - Mothers do not understand the instructions. -Mothers do not have financial resources to get to the health center.

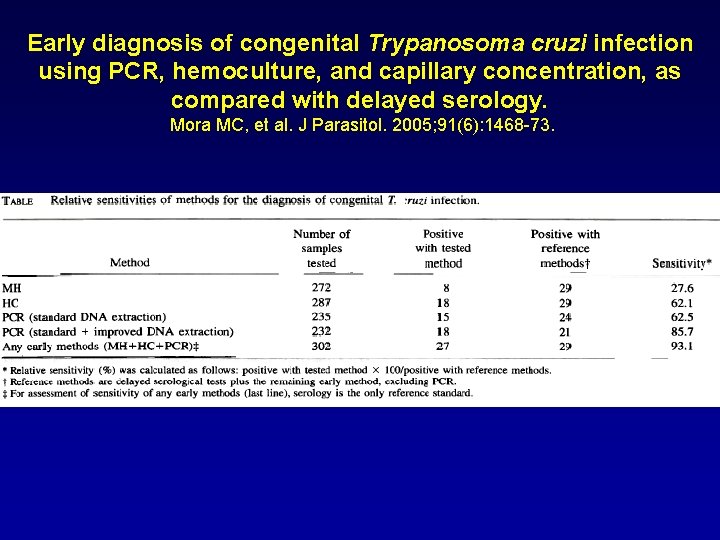
Early diagnosis of congenital Trypanosoma cruzi infection using PCR, hemoculture, and capillary concentration, as compared with delayed serology. Mora MC, et al. J Parasitol. 2005; 91(6): 1468 -73.

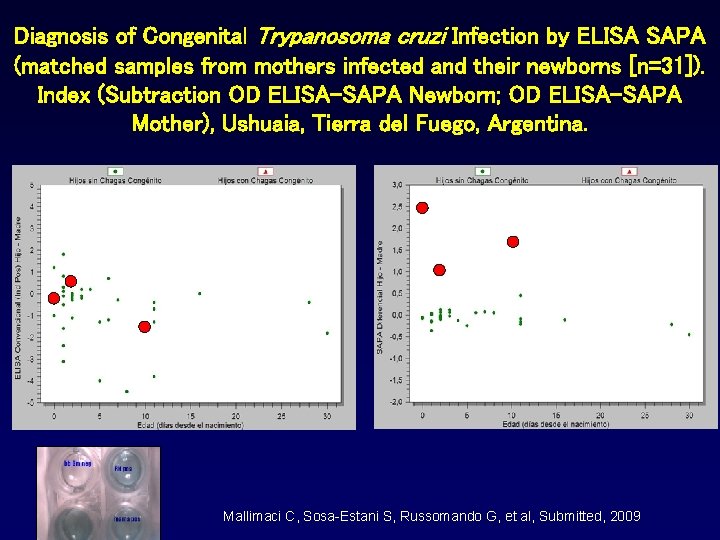
Diagnosis of Congenital Trypanosoma cruzi Infection by ELISA SAPA (matched samples from mothers infected and their newborns [n=31]). Index (Subtraction OD ELISA-SAPA Newborn; OD ELISA-SAPA Mother), Ushuaia, Tierra del Fuego, Argentina. Mallimaci C, Sosa-Estani S, Russomando G, et al, Submitted, 2009

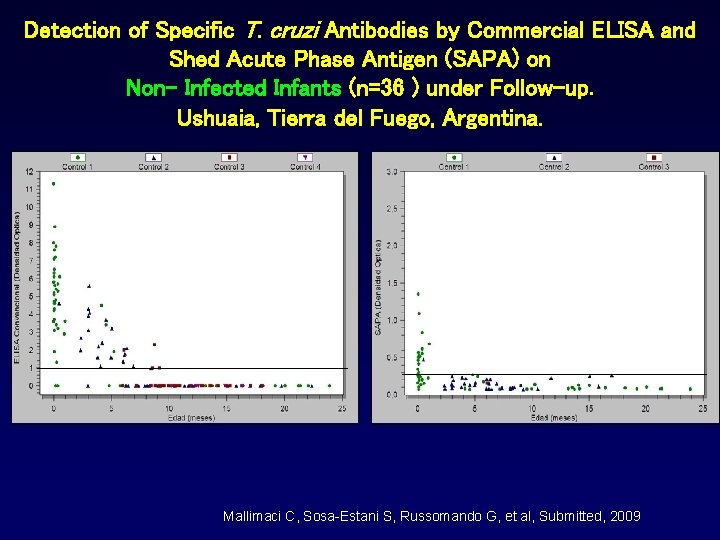
Detection of Specific T. cruzi Antibodies by Commercial ELISA and Shed Acute Phase Antigen (SAPA) on Non- Infected Infants (n=36 ) under Follow-up. Ushuaia, Tierra del Fuego, Argentina. Mallimaci C, Sosa-Estani S, Russomando G, et al, Submitted, 2009

Evaluation of technology – Implementation research • New tools must to be addressed to the PHC System • Main user National Programs, Public Health S • Wide range of beneficiaries Photo: H Freilij. Bs. As, ARG Photo: S Sosa-Estani, Las Lomitas, Formosa, ARG

They are waiting for: • the researcher to research, • the politician to decide, • and the health worker to act
 Take-home message examples
Take-home message examples Doença de chagas
Doença de chagas Oncocercose
Oncocercose Sintomas fase aguda doença de chagas
Sintomas fase aguda doença de chagas Instituto evandro chagas
Instituto evandro chagas Disenteria amebiana
Disenteria amebiana International symposium on molecular spectroscopy
International symposium on molecular spectroscopy International police executive symposium
International police executive symposium International perforating symposium
International perforating symposium Ips perforating
Ips perforating International perforating symposium
International perforating symposium International perforating symposium
International perforating symposium International perforating symposium
International perforating symposium Chara screenshot
Chara screenshot Communicable disease and non communicable disease
Communicable disease and non communicable disease Who functions
Who functions Thẻ vin
Thẻ vin Tư thế ngồi viết
Tư thế ngồi viết Bàn tay mà dây bẩn
Bàn tay mà dây bẩn Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Bổ thể
Bổ thể Tư thế ngồi viết
Tư thế ngồi viết