CHAGAS DISEASE Overwiev Diagnosis Proposals for WHO Ref






















- Slides: 36

CHAGAS DISEASE Overwiev Diagnosis Proposals for WHO Ref. Preparation Alejandro O. Luquetti Laboratório de Pesquisa em Doença de Chagas Hospital das Clínicas e Instituto de Patologia Tropical e Saúde Pública, Universidade Federal de Goiás Brasil

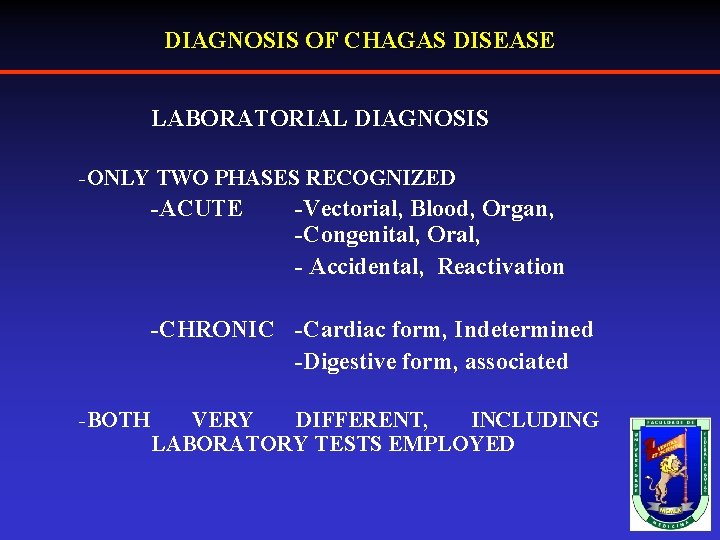
DIAGNOSIS OF CHAGAS DISEASE LABORATORIAL DIAGNOSIS -ONLY TWO PHASES RECOGNIZED -ACUTE -Vectorial, Blood, Organ, -Congenital, Oral, - Accidental, Reactivation -CHRONIC -Cardiac form, Indetermined -Digestive form, associated -BOTH VERY DIFFERENT, INCLUDING LABORATORY TESTS EMPLOYED

DIAGNOSIS OF CHAGAS DISEASE DIAGNOSIS (Acute and chronic phase) A)EPIDEMIOLOGY B)CLINICAL FINDINGS C)LABORATORY TESTS -All should fit

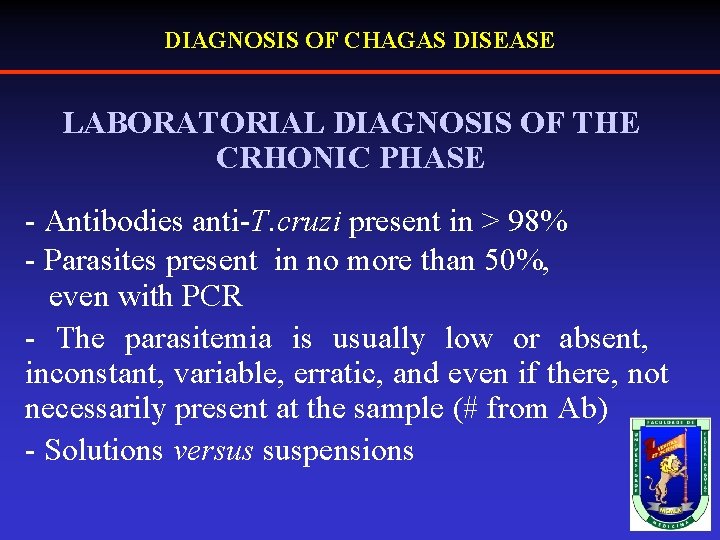
DIAGNOSIS OF CHAGAS DISEASE LABORATORIAL DIAGNOSIS OF THE CRHONIC PHASE - Antibodies anti-T. cruzi present in > 98% - Parasites present in no more than 50%, even with PCR - The parasitemia is usually low or absent, inconstant, variable, erratic, and even if there, not necessarily present at the sample (# from Ab) - Solutions versus suspensions

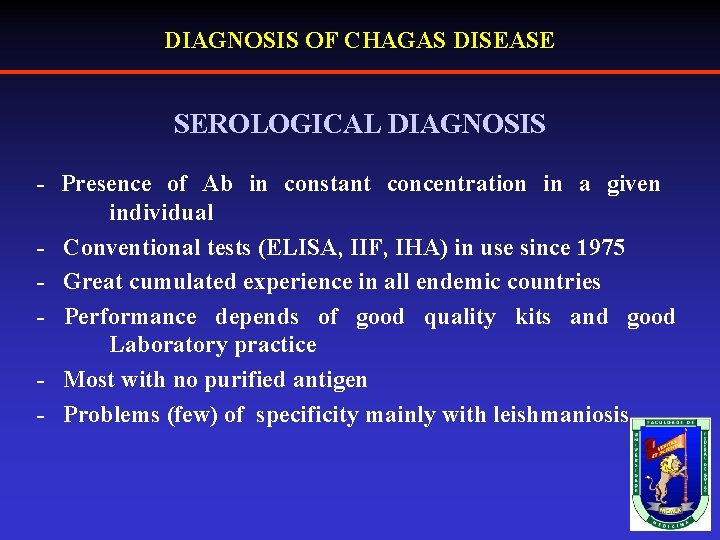
DIAGNOSIS OF CHAGAS DISEASE SEROLOGICAL DIAGNOSIS - Presence of Ab in constant concentration in a given individual - Conventional tests (ELISA, IIF, IHA) in use since 1975 - Great cumulated experience in all endemic countries - Performance depends of good quality kits and good Laboratory practice - Most with no purified antigen - Problems (few) of specificity mainly with leishmaniosis

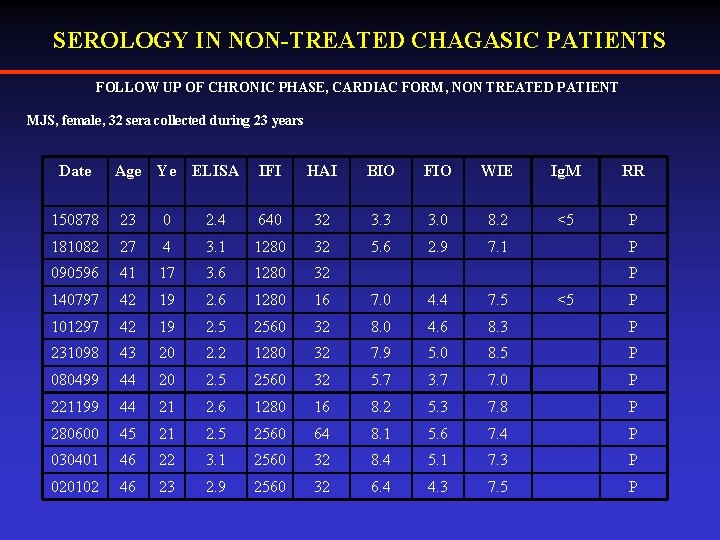
SEROLOGY IN NON-TREATED CHAGASIC PATIENTS FOLLOW UP OF CHRONIC PHASE, CARDIAC FORM, NON TREATED PATIENT MJS, female, 32 sera collected during 23 years Date Age Ye ELISA IFI HAI BIO FIO WIE Ig. M RR <5 P 150878 23 0 2. 4 640 32 3. 3 3. 0 8. 2 181082 27 4 3. 1 1280 32 5. 6 2. 9 7. 1 090596 41 17 3. 6 1280 32 140797 42 19 2. 6 1280 16 7. 0 4. 4 7. 5 101297 42 19 2. 5 2560 32 8. 0 4. 6 8. 3 P 231098 43 20 2. 2 1280 32 7. 9 5. 0 8. 5 P 080499 44 20 2. 5 2560 32 5. 7 3. 7 7. 0 P 221199 44 21 2. 6 1280 16 8. 2 5. 3 7. 8 P 280600 45 21 2. 5 2560 64 8. 1 5. 6 7. 4 P 030401 46 22 3. 1 2560 32 8. 4 5. 1 7. 3 P 020102 46 23 2. 9 2560 32 6. 4 4. 3 7. 5 P P P <5 P

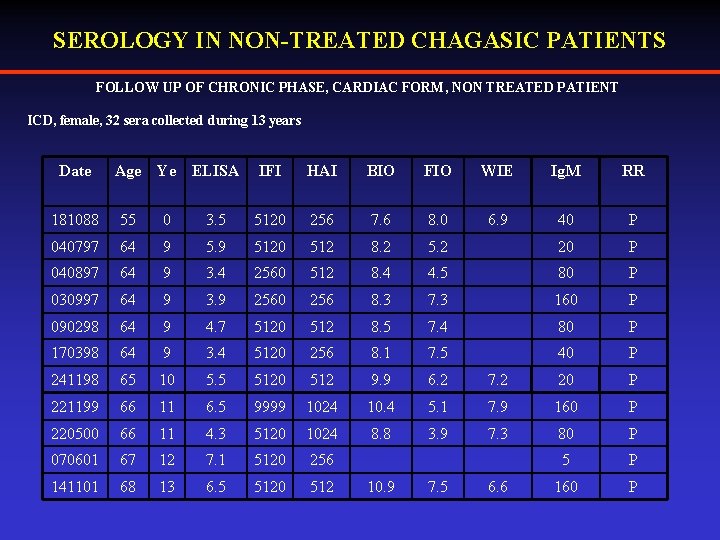
SEROLOGY IN NON-TREATED CHAGASIC PATIENTS FOLLOW UP OF CHRONIC PHASE, CARDIAC FORM, NON TREATED PATIENT ICD, female, 32 sera collected during 13 years Date Age Ye ELISA IFI HAI BIO FIO WIE Ig. M RR 6. 9 40 P 181088 55 0 3. 5 5120 256 7. 6 8. 0 040797 64 9 5120 512 8. 2 5. 2 20 P 040897 64 9 3. 4 2560 512 8. 4 4. 5 80 P 030997 64 9 3. 9 2560 256 8. 3 7. 3 160 P 090298 64 9 4. 7 5120 512 8. 5 7. 4 80 P 170398 64 9 3. 4 5120 256 8. 1 7. 5 40 P 241198 65 10 5. 5 5120 512 9. 9 6. 2 7. 2 20 P 221199 66 11 6. 5 9999 1024 10. 4 5. 1 7. 9 160 P 220500 66 11 4. 3 5120 1024 8. 8 3. 9 7. 3 80 P 070601 67 12 7. 1 5120 256 5 P 141101 68 13 6. 5 5120 512 160 P 10. 9 7. 5 6. 6

SEROLOGICAL DIAGNOSIS OF T. cruzi INFECTION THE QUESTION OF T. cruzi I and II & SEROLOGY -As known: T. cruzi I: homogeneous, north Amazonas river, Rhodn. isolated humans and silvatic, didelphis, palm trees, also sylvatic below Amazonas, some humans double infection, only appears on immunosupression or tissues, easy treatment T. cruzi II: b: homogeneous, humans east Brazil, associated megaesophagus, low congenital(<1%), severe cardiopathy, difficult treatm. Years Neg. a: silvatic, armadillo, soil(Z 3), P. genic. c: hybrid? d & e: hybrids: south BR, AR, UR, PY, BO, CH, easy response treatment, incl. Nf congenital 5%, megacolon & card.

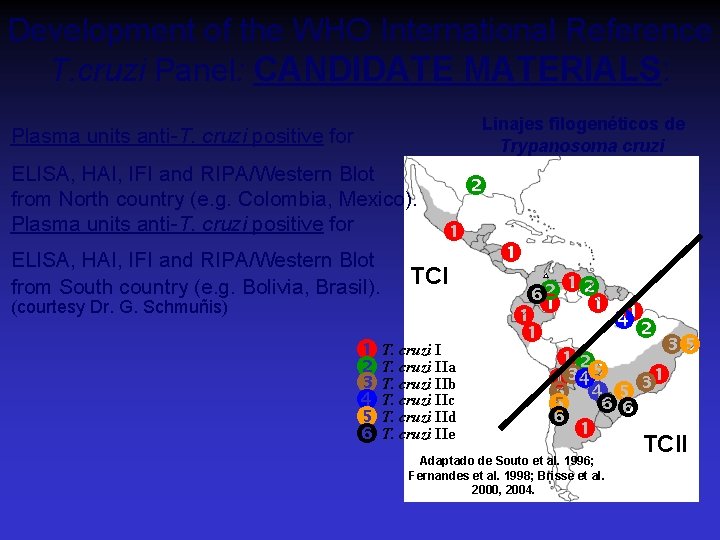
Development of the WHO International Reference T. cruzi Panel: CANDIDATE MATERIALS: Linajes filogenéticos de Trypanosoma cruzi Plasma units anti-T. cruzi positive for ELISA, HAI, IFI and RIPA/Western Blot from North country (e. g. Colombia, Mexico). Plasma units anti-T. cruzi positive for ELISA, HAI, IFI and RIPA/Western Blot from South country (e. g. Bolivia, Brasil). TCI (courtesy Dr. G. Schmuñis) I T. T. cruzi IIa T. cruzi IIb T. cruzi IIc T. cruzi IId T. cruzi IIe TCII Adaptado de Souto et al. 1996; Fernandes et al. 1998; Brisse et al. 2000, 2004.

SEROLOGICAL DIAGNOSIS OF T. cruzi INFECTION THE QUESTION OF T. cruzi I & II AND SEROLOGY -A: T. cruzi II and reagents made from T. cruzi II: ok -B: T. cruzi II and reagents made from T. c I not extensively tested Our experience (IFI, ELISA) strain Queretaro: similar results (? ) -C: T. cruzi I and reagents made from T. c. I: ok (MX, CO) -D: T. cruzi I and reagents made from T. c. II: some (MX, HN, CO, VE) ok other (MX, CO) low reactivity some sera Our experience: ok (MX, HN, CO, VZ, )

SEROLOGICAL DIAGNOSIS OF T. cruzi INFECTION DIAGNOSIS IN EACH SITUATION 1)DIAGNOSIS OF A PATIENT - Send by physician, for clarify etiology - Select tests with higher specificity - Avoid false positive, psychological and/or legal consequences - Use two tests of different principles, conventional or one conventional and the other rapid, or 1 ELISA crude + ELISA recomb. - Valorize titers - If both positive, at higher titers, result could be signed

CHAGAS DISEASE DIAGNOSIS OF INFECTION BY T. cruzi IN DIFFERENT CONTEXTS 2) DONOR EXCLUSION - Blood bank: need to offer a good quality product - Use of kits with high sensitivity - Avoid false-negative, legal consequences - Use of two tests of different principle, convencional one should be ELISA + IIF or IHA or rapid test - Use one test, ELISA, only if a proper external quality control exist - If both NEGATIVE, blood may be used

LABORATOY DIAGNOSIS OF T. cruzi INFECTION • RELIABLE RESULTS IN SEROLOGY – Matherials: approved kit, retested for lot at the lab(internal panel with low positives and high negatives • Good laboratory practices: temperatures, p. H, etc. • POP : description of each procedure, in detail – Methods: Programs of technical training (Telelab) – Quality: Lab ought to participate in an External Quality Control Programme, provided that: • This programm send at least 2 panels/year • The Lab should be approved

LABORATOY DIAGNOSIS OF T. cruzi INFECTION • PROGRAMS of EXTERNAL QUALITY CONTROL – Initial difficulty to mount serum panels (AR/CH/BR/PY) Meeting OPS-BH 1994) – Difficult to obtain panels in non endemic countries – Initiative of OPS/PANEL São Paulo (BR) 1995 – Programm operative in >18 countries – Priority in reccommendations of South Cone (1999) – Programms of Hemotherapy Societies (AR, BR) – Programm of MPH, BR, COSAH>ANVISA (2001)

LABORATOY DIAGNOSIS OF T. cruzi INFECTION • CONTROL QUALITY PROGRAM OF THE MINISTRY OF HEALTH, BR FOR EXTERNAL CONTROL IN BLOOD BANKS – – – – – Joint venture National Agency of Sanitary Surveill. /Fiocruz Both belong to Ministery of Health, coordination, execution Technical Committee, 1 by area (syphilis, HIV, etc) Several meetings/year, evaluation of results by marker Three panels/year (6 x 3) – by post Started on 2000, processed 2. 718 plasma bags (2005) From 2001 to 2008, 19 evaluations, of 135 services (90% public) Increase in results, from 3, 6 % to 0, 9% discordances. ) Indirectly detected problems with different lots of used kits

LABORATOY DIAGNOSIS OF T. cruzi INFECTION • EVALUATION OF ELISA KITS AVAILABLE IN BRAZIL – Study performed by Lab Coordination, Min. Health – Bought all certified kits available in Brazil (n=12) – Selection of 152 sera (half negative) – Blind tests by 4 labs: MG, PE, MS, GO – Kappa index of 0, 71 to 0, 98. Sensitivity 0, 97 a 1, 0 – 6 kits sensitivity = 1, 0. 5 kits = 0, 99. 1 kit =0, 97. – Trades: Adaltis, Bioma, Biomerieux, Bioschil, Biozima, – Ebram, Hemagen, Omega, REM, Wama, Wiener – This study allows to exclude some trades, based on published data (www.

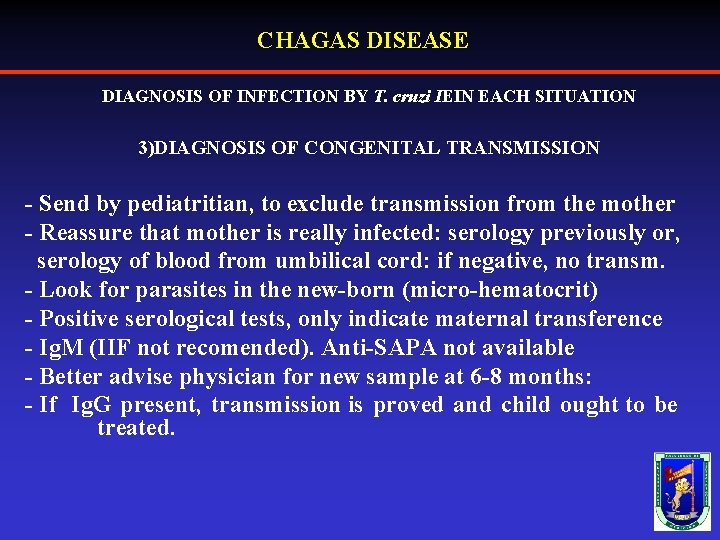
CHAGAS DISEASE DIAGNOSIS OF INFECTION BY T. cruzi IEIN EACH SITUATION 3)DIAGNOSIS OF CONGENITAL TRANSMISSION - Send by pediatritian, to exclude transmission from the mother - Reassure that mother is really infected: serology previously or, serology of blood from umbilical cord: if negative, no transm. - Look for parasites in the new-born (micro-hematocrit) - Positive serological tests, only indicate maternal transference - Ig. M (IIF not recomended). Anti-SAPA not available - Better advise physician for new sample at 6 -8 months: - If Ig. G present, transmission is proved and child ought to be treated.

SITUAÇÃO ATUAL DA DOENÇA DE CHAGAS SEROLOGIA EN LACTANTES DE MADRES INFECTADAS -RESULTADOS OBTENIDOS EN 56 LACTANTES - Suero del primer mes: títulos muy elevados, similares a los de la madre: (n=17) IFI 1. 280 Elisa: 2, 6 HAI 256 Pa. GIA pos - Suero del segundo mes: IFI 320 Elisa: 1, 5 HAI 16 Pa. GIA variab - Suero del tercer mes: IFI 80 Elisa: 1, 2 HAI 8 Pa. GIA variab - Suero del cuarto mes: IFI 40 Elisa: 0, 6 HAI 4 Pa. GIA neg - Suero del 6 o. mes(n=13): IFI 10 a 40 Elisa: 0, 6 HAI <4 Pa. Gia neg - Suero del 7 o. mes(n=9): IFI <10 Elisa 0, 4 HAI <2 Pa. GIA neg - Suero del 8 o. mes(n=8): IFI<10 Elisa 0, 4 HAI<2 Pa. GIA negativo

CHAGAS DISEASE DIAGNOSIS OF INFECTION BY T. cruzi IN EACH CONTEXT 4) FOLLOW UP OF A SPECIFICALLY TREATED - Send by physician, looking for cure - Cure is possible in all newborns, 60% of acute phase and children and up to 25% of adults, after benznidazol x 60 days - Cure criteria is abscence of antibodies, formerly present - Time to attain: months in congenital, years in acute/children and decades in adults - For these, need to preserve previous sera with glicerol - To procede in parallel com os soros anteriores e o atual

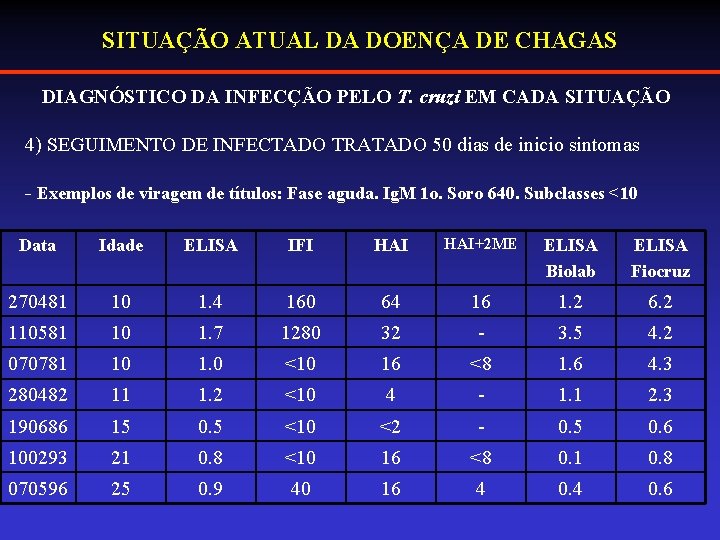
SITUAÇÃO ATUAL DA DOENÇA DE CHAGAS DIAGNÓSTICO DA INFECÇÃO PELO T. cruzi EM CADA SITUAÇÃO 4) SEGUIMENTO DE INFECTADO TRATADO 50 dias de inicio sintomas - Exemplos de viragem de títulos: Fase aguda. Ig. M 1 o. Soro 640. Subclasses <10 Data Idade ELISA IFI HAI+2 ME ELISA Biolab ELISA Fiocruz 270481 10 1. 4 160 64 16 1. 2 6. 2 110581 10 1. 7 1280 32 - 3. 5 4. 2 070781 10 1. 0 <10 16 <8 1. 6 4. 3 280482 11 1. 2 <10 4 - 1. 1 2. 3 190686 15 0. 5 <10 <2 - 0. 5 0. 6 100293 21 0. 8 <10 16 <8 0. 1 0. 8 070596 25 0. 9 40 16 4 0. 6

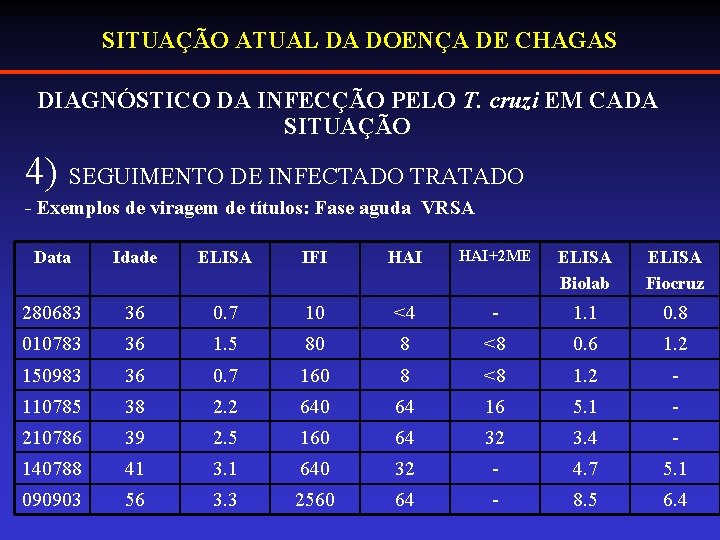
SITUAÇÃO ATUAL DA DOENÇA DE CHAGAS DIAGNÓSTICO DA INFECÇÃO PELO T. cruzi EM CADA SITUAÇÃO 4) SEGUIMENTO DE INFECTADO TRATADO - Exemplos de viragem de títulos: Fase aguda VRSA Data Idade ELISA IFI HAI+2 ME ELISA Biolab ELISA Fiocruz 280683 36 0. 7 10 <4 - 1. 1 0. 8 010783 36 1. 5 80 8 <8 0. 6 1. 2 150983 36 0. 7 160 8 <8 1. 2 - 110785 38 2. 2 640 64 16 5. 1 - 210786 39 2. 5 160 64 32 3. 4 - 140788 41 3. 1 640 32 - 4. 7 5. 1 090903 56 3. 3 2560 64 - 8. 5 6. 4

PROPOSAL OF A REFERENCE PANEL FOR CHAGAS WHO INTERNATIONAL BIOLOGICAL REFERENCE PREPARATION - Biological standard: measure concentration of substance that cannot be characterised by chemical and physical methods -International standard: preparation to which an International Unit has been assigned - WHO holds a number of reference sera for several diseases (syphilis, hepatitis, etc. ). Also, vaccines, toxoids. Chagas is not included. -Expert Committee on Biological Standardization meets once a year. Each preparation is analised, as well as candidates and substitutions need to be aproved.

PROPOSAL OF A REFERENCE PANEL FOR CHAGAS WHO INTERNATIONAL BIOLOGICAL REFERENCE PREPARATION - The Expert Committee on Biological Standardization, note the absence of a Biological standard (serum) on Chagas disease (Dr. Padilla). - A WHO Consultation on International Biological reference preparations for Chagas diagnostic tests was launched. - This meeting was held previously to the annual meeting, from 2 to 3 July 2007, in Geneve, with 33 participants (half specialists) and 4 industries. - After 2 days of presentations and discussion, a Coordinating Group was elected with AL, Brazil as Chairman, Carmen Guzmán, Mexico and Marcia Otani, São Paulo) to support the development of the reference panel. -After some preliminary experiments, the proposals of the group were presented by the chairman at the meeting of the Expert Committe, 8 -12 october, 2007.

PROPOSAL OF A REFERENCE PANEL FOR CHAGAS WHAT WE NEED? Some misunderstanig on the circuntances a reference serum is employed, aroused. STEPS ON THE NEED FOR A RELIABLE PREPARATION. WHAT FOR? - Any organization working in diagnosis of an infectious disease need to have a suitable, reliable, serum, from a surely infected patient, the same used in different countries. -A) idea (thinkers): university/foundation: creates a diagnostic test in a support, with selected antigens and get good results > publication (“in house” phase). -B) building (makers): an industry start to invest and gets a reliable test, with reproducible results. -C) application (users): blood banks/diagnostic labs buy the product (kit) and get reliable and repetitive results -B and C need reliable sera for quality control

PROPOSAL OF A REFERENCE PANEL FOR CHAGAS WHAT WE NEED? KINDS OF REFERENCE SERA AND PANEL OF SERA CHARACT. INTERNATIONAL PANEL OF PANEL FOR REFERENCE SERA FOR EXTERNAL SERUM TEST KITS QUALITY CONTROL --------------------------------------------------------Nr. samples 01 200 6/panel Volume/aliquot 0. 5/1. 0 m. L Renewal each 5 years 1 year each 4 months Nr. samples/5 y. 1 1. 000 6 x 3 x 5 y=90 Total volume 2/3 L/sample 2 -5 m. L If 100 labs=100 m. L/each Origin of serum blood donors may be patients blood donors Mixtures needed, few not recommend. Not recommended Strenght medium mixed(high+low) medium Etical issues informed consent ---------------------------------------------------------

PROPOSAL OF A REFERENCE PANEL FOR CHAGAS ACCORDING TO RECCOMENDATIONS OF THE CONSULTATION GROUP - Need for two Reactive sera. Tc. I & Tc. II. - Need for a negative control. Check if exists for other diseases. Perhaps a naive requirement - Consensus in the antibody concentration, for both reactive. Should be relatively low, not high. - In a scale (IFI) from 1/80 to 1/10. 240, 160 -640 - In ELISA, O. D. 2 – 2 ½ higher than cut-off - Need 2 different sources, i. e. Mexico & Brazil

PROPOSAL OF A REFERENCE PANEL FOR CHAGAS ACCORDING TO RECCOMENDATIONS OF THE CONSULTATION GROUP - Need 1. 5 – 3. 0 L (3. 000 vials with 0, 5 – 1, 0 m. L/each. - Operational and etical problems, if from single, infected. - More feasible from a pool of few positives. - Alternatively, (or/and) start from high responder donor, dilute with negative, look for end point. - Explore both, pool of high titer, then diluted

PROPOSAL OF A REFERENCE PANEL FOR CHAGAS ACCORDING TO RECCOMENDATIONS OF THE CONSULTATION GROUP - First issue: is possible to dilute a high ab concentration sera, and, until what dilution, without loose titer? - A well doccumented serum from an infected another from non-infected were experimented, as follows: - 1: 1 (final dilution 1/2, i. e. 100 u. L posit. + 100 u. L negative) - 1: 2; 1: 3, 1: 4, 1: 5, 1: 10, 1: 20, 1: 50, 1: 100 - results showed clearly positive up to 1: 5 - 1: 10. - higher dilutions were not possible to use.

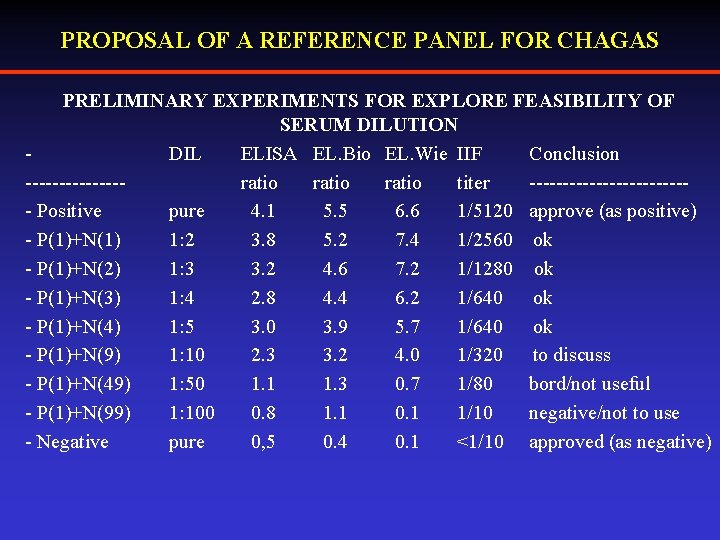
PROPOSAL OF A REFERENCE PANEL FOR CHAGAS PRELIMINARY EXPERIMENTS FOR EXPLORE FEASIBILITY OF SERUM DILUTION DIL ELISA EL. Bio EL. Wie IIF Conclusion -------ratio titer ------------ Positive pure 4. 1 5. 5 6. 6 1/5120 approve (as positive) - P(1)+N(1) 1: 2 3. 8 5. 2 7. 4 1/2560 ok - P(1)+N(2) 1: 3 3. 2 4. 6 7. 2 1/1280 ok - P(1)+N(3) 1: 4 2. 8 4. 4 6. 2 1/640 ok - P(1)+N(4) 1: 5 3. 0 3. 9 5. 7 1/640 ok - P(1)+N(9) 1: 10 2. 3 3. 2 4. 0 1/320 to discuss - P(1)+N(49) 1: 50 1. 1 1. 3 0. 7 1/80 bord/not useful - P(1)+N(99) 1: 100 0. 8 1. 1 0. 1 1/10 negative/not to use - Negative pure 0, 5 0. 4 0. 1 <1/10 approved (as negative)

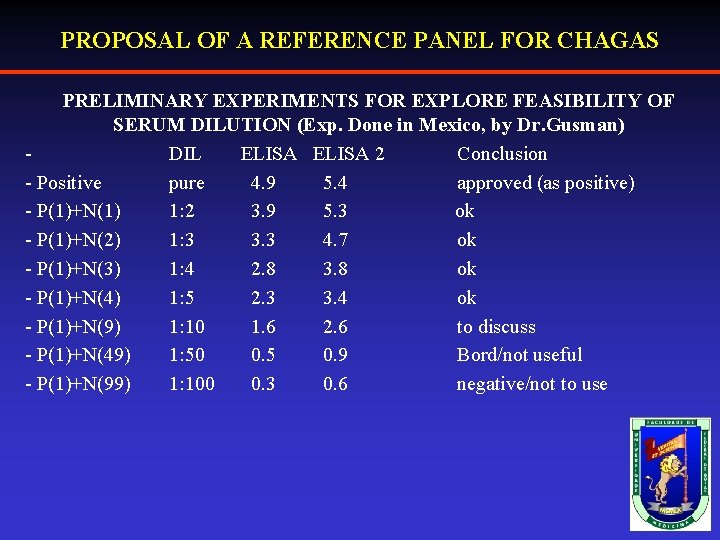
PROPOSAL OF A REFERENCE PANEL FOR CHAGAS PRELIMINARY EXPERIMENTS FOR EXPLORE FEASIBILITY OF SERUM DILUTION (Exp. Done in Mexico, by Dr. Gusman) DIL ELISA 2 Conclusion - Positive pure 4. 9 5. 4 approved (as positive) - P(1)+N(1) 1: 2 3. 9 5. 3 ok - P(1)+N(2) 1: 3 3. 3 4. 7 ok - P(1)+N(3) 1: 4 2. 8 3. 8 ok - P(1)+N(4) 1: 5 2. 3 3. 4 ok - P(1)+N(9) 1: 10 1. 6 2. 6 to discuss - P(1)+N(49) 1: 50 0. 5 0. 9 Bord/not useful - P(1)+N(99) 1: 100 0. 3 0. 6 negative/not to use

PROPOSAL OF A REFERENCE PANEL FOR CHAGAS BRAZILIAN SELECTION OF SAMPLES FOR PREPARATION OF WHO REF. SERA - All samples from Hemocentro, São Paulo, HC, USP. - Selection performed by one member of the coordinating group, Dr. M. Otani - 4 plasmas selected primarily -Identification, date collection, date born, place/locality sex, epidemiology -A) 103. 350. 016, - 190307, 160850, male, residence SP. La Canela (Chile),

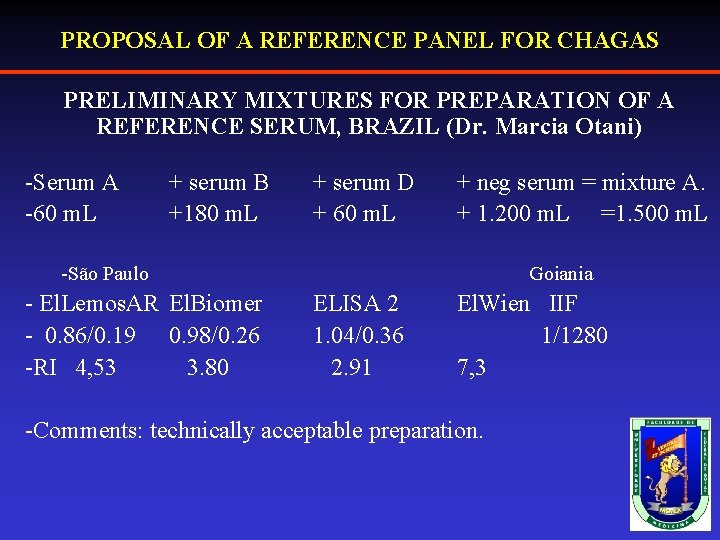
PROPOSAL OF A REFERENCE PANEL FOR CHAGAS PRELIMINARY MIXTURES FOR PREPARATION OF A REFERENCE SERUM, BRAZIL (Dr. Marcia Otani) -Serum A -60 m. L + serum B +180 m. L + serum D + 60 m. L + neg serum = mixture A. + 1. 200 m. L =1. 500 m. L -São Paulo - El. Lemos. AR El. Biomer - 0. 86/0. 19 0. 98/0. 26 -RI 4, 53 3. 80 Goiania ELISA 2 1. 04/0. 36 2. 91 El. Wien IIF 1/1280 7, 3 -Comments: technically acceptable preparation.

PROPOSAL OF A REFERENCE PANEL FOR CHAGAS NEXT STEPS FOR A REFERENCE PANEL OF SERA (2007) (presented to the Expert Comittee on Biological Standardization, October, 2007) - Mexico is planning to send the 1, 5 L of a pool of 5 donors in nov. - To discuss the need for a negative control for endemic area - To re-check for absence of HIV, hepatitis B, other markers - To check each one of the mixtures with IHA, different trades of IIF - To check with ELISAS available in BR, AR, PY, CH, MX. - To check results with rapid tests available - To check for Reumatoid Factor and Ig. M anti-T. cruzi - To send pools for liophilization. - To distribute samples to different industries/manufacturers x analysis

PROPOSAL OF A REFERENCE PANEL FOR CHAGAS NEXT STEPS FOR A REFERENCE PANEL OF SERA (2008) -WHERE WE ARE? (OCTOBER 2008) - After one year of presentation to the Expert Committee: - Mexico made progress by sending to São Paulo the 1, 5 L of serum from several donors. - Samples are frozen down, waiting orders/authorization from WHO/Geneve. - Due to administrative problems in WHO, the meeting of Chagas was posponed. - Due to the same problems the Expert Committee was held in october, no Chagas -NEXT STEPS: - To check preparations with ELISAS available in BR, AR, PY, CH, MX, US. - To check results with rapid tests available -To send pools for liophilization. - To distribute samples to different industries/manufacturers for analysis

PROPOSAL OF A REFERENCE PANEL FOR CHAGAS FINALLY, WHAT DO WE NEED? - A lyophylized batch of aprox. 1, 500 aliquots of serum, with 0. 5 – 1. 0 m. L each, with antibodies anti-T. cruzi in such a concentration that most/all available, good quality reagents prepared with T. cruzi 2 Ag will be able to detect. This serum will be prepared from T. cruzi 2 patients. - To prepare a similar batch from sera from donors of T. c 1 area - As these preparations may be used for calibration of reagents, they should not have high concentration of Ab, but enough to allow their detection by most already known kits, without further manipulation. - We will not prepare panels of sera, but a single pair of reference sera - Even if some wanted to have also a negative control, we do not agree - These preparations will be available to all those who need a stable and reliable sample of serum with antibodies against T. cruzi

PROPOSAL OF A REFERENCE PANEL FOR CHAGAS FINALLY, WHAT DO WE NEED? FOR WHOM? - These preparations will be available to all those who need a stable and reliable sample of serum with antibodies against T. cruzi (under WHO regulations) - 1) Blood banks, to calibrate their reagents and prepare a panel -2) Diagnosis labs, for same purposes -3) Manufacturers, to have a reference sample to test against new panels of sera. -(suggested use: 2 – 3 times / year, together with internal controls, when new reagents are employed or new lots)
 Sintomas fase aguda doença de chagas
Sintomas fase aguda doença de chagas Instituto evandro chagas
Instituto evandro chagas Leishmaniose
Leishmaniose Take home message
Take home message Chagas
Chagas Vetor chagas
Vetor chagas Medical diagnosis and nursing diagnosis difference
Medical diagnosis and nursing diagnosis difference Nursing process objectives
Nursing process objectives Definition of nursing diagnosis
Definition of nursing diagnosis Perbedaan diagnosis gizi dan diagnosis medis
Perbedaan diagnosis gizi dan diagnosis medis Medical diagnosis and nursing diagnosis difference
Medical diagnosis and nursing diagnosis difference Nursing diagnosis for meniere's disease
Nursing diagnosis for meniere's disease Behcet disease diagnosis
Behcet disease diagnosis Diagnosis of periodontal disease
Diagnosis of periodontal disease Nursing diagnosis for vision impairment
Nursing diagnosis for vision impairment Solicited internal
Solicited internal Eclipse computing proposals slow
Eclipse computing proposals slow Writing and completing reports and proposals
Writing and completing reports and proposals Fire insurance contract
Fire insurance contract Delhi muslim proposals
Delhi muslim proposals Proposals and formal reports
Proposals and formal reports When evaluating cost-cutting proposals
When evaluating cost-cutting proposals Writing thesis and dissertation proposals
Writing thesis and dissertation proposals Artificial intelligence thesis proposals
Artificial intelligence thesis proposals Developing effective research proposals
Developing effective research proposals Reports and proposals
Reports and proposals Bharathi viswanathan
Bharathi viswanathan Mekanisme debit dan kredit
Mekanisme debit dan kredit Laporan perubahan modal
Laporan perubahan modal Simon and garfunkel gay
Simon and garfunkel gay Hockey ref positioning
Hockey ref positioning Ref-qualified member functions
Ref-qualified member functions Packages_display.php?ref=
Packages_display.php?ref= Twitter ref 2021
Twitter ref 2021 Ref 2021 twitter
Ref 2021 twitter Ref
Ref Ref 2026
Ref 2026