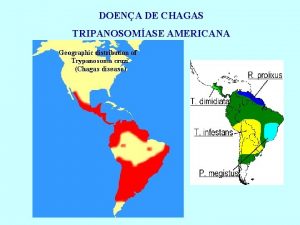
CHAGAS DISEASE Serological Diagnosis and the humoral immune













- Slides: 29

CHAGAS DISEASE Serological Diagnosis and the humoral immune response after specific treatment Alejandro O. Luquetti & Anis Rassi Laboratório de Pesquisa em Doença de Chagas Hospital das Clínicas e Instituto de Patologia Tropical e Saúde Pública, Universidade Federal de Goiás Brasil

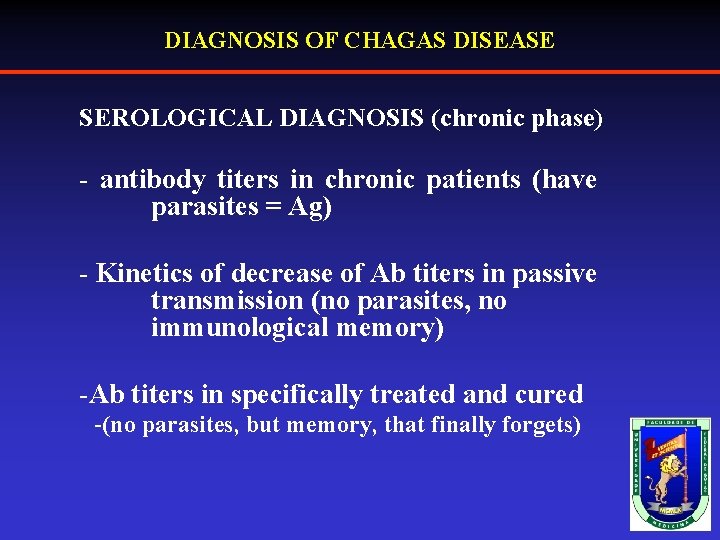
DIAGNOSIS OF CHAGAS DISEASE SEROLOGICAL DIAGNOSIS (chronic phase) - antibody titers in chronic patients (have parasites = Ag) - Kinetics of decrease of Ab titers in passive transmission (no parasites, no immunological memory) -Ab titers in specifically treated and cured -(no parasites, but memory, that finally forgets)

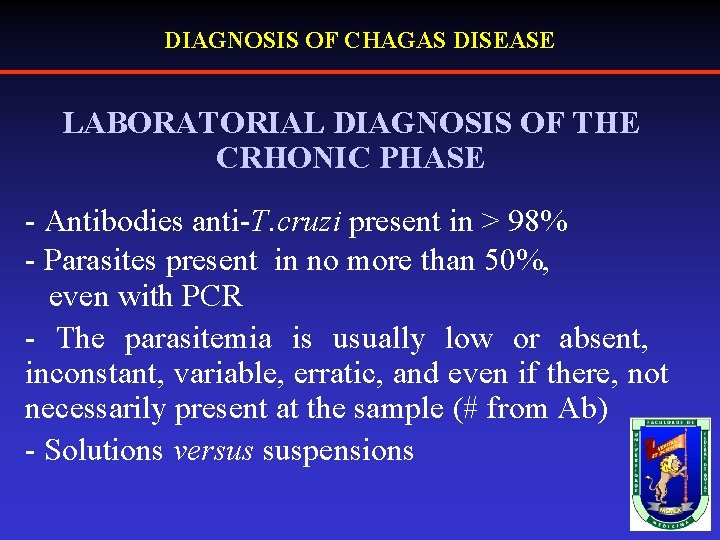
DIAGNOSIS OF CHAGAS DISEASE LABORATORIAL DIAGNOSIS OF THE CRHONIC PHASE - Antibodies anti-T. cruzi present in > 98% - Parasites present in no more than 50%, even with PCR - The parasitemia is usually low or absent, inconstant, variable, erratic, and even if there, not necessarily present at the sample (# from Ab) - Solutions versus suspensions

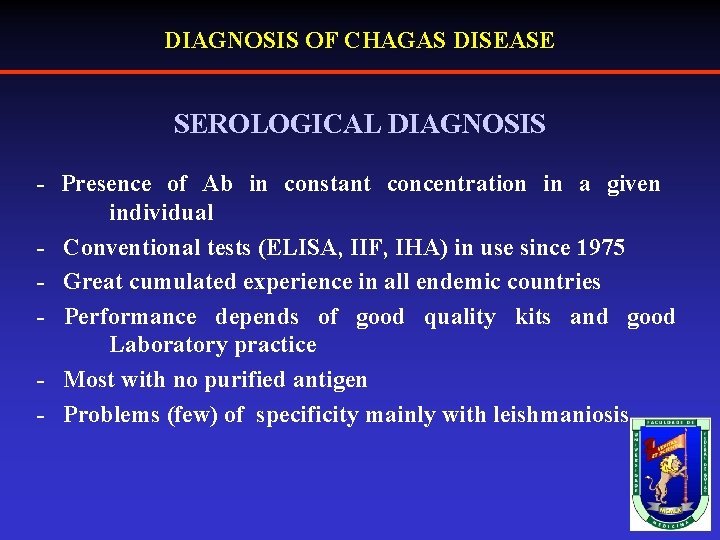
DIAGNOSIS OF CHAGAS DISEASE SEROLOGICAL DIAGNOSIS - Presence of Ab in constant concentration in a given individual - Conventional tests (ELISA, IIF, IHA) in use since 1975 - Great cumulated experience in all endemic countries - Performance depends of good quality kits and good Laboratory practice - Most with no purified antigen - Problems (few) of specificity mainly with leishmaniosis

DIAGNOSIS OF CHAGAS DISEASE SEROLOGICAL DIAGNOSIS TO OBTAIN RELIABLE RESULTS 1. Serum in good conditions (glycerol 50%). 2. (bad storage (4º. C) low titers) 3. 2. Comercial kits: ought to use (no in house) a)select a good performance one b)chech reactivity of lot c)use several internal controls d)follow strictly technical instructions 3. Good laboratory practices 4. Facilitate quiet environment, to avoid technical errors

DIAGNOSIS OF CHAGAS DISEASE SEROLOGICAL DIAGNOSIS - If properly done, allowed differences are of one titer, with same serum, different days - Very precise tool (if comercially acquired) - All in duplicate in different zone of plate - If duplicates do not fit, repeat (i. e. 1. 5 - 2. 7) - Once system is working, go ahead

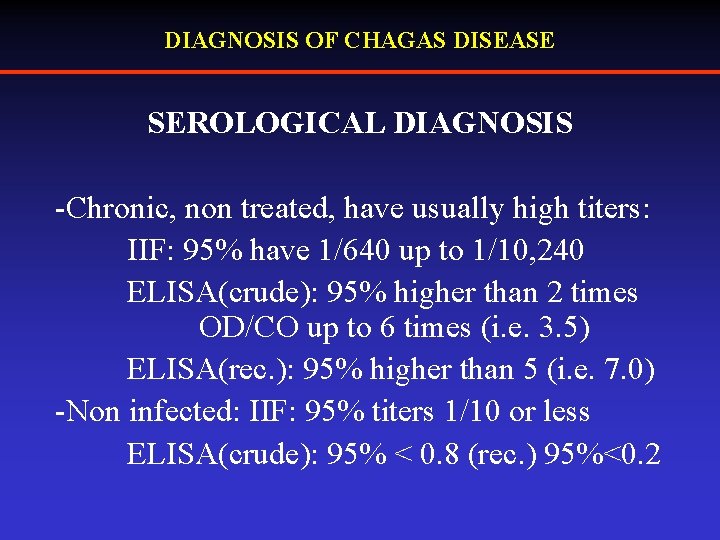
DIAGNOSIS OF CHAGAS DISEASE SEROLOGICAL DIAGNOSIS -Chronic, non treated, have usually high titers: IIF: 95% have 1/640 up to 1/10, 240 ELISA(crude): 95% higher than 2 times OD/CO up to 6 times (i. e. 3. 5) ELISA(rec. ): 95% higher than 5 (i. e. 7. 0) -Non infected: IIF: 95% titers 1/10 or less ELISA(crude): 95% < 0. 8 (rec. ) 95%<0. 2

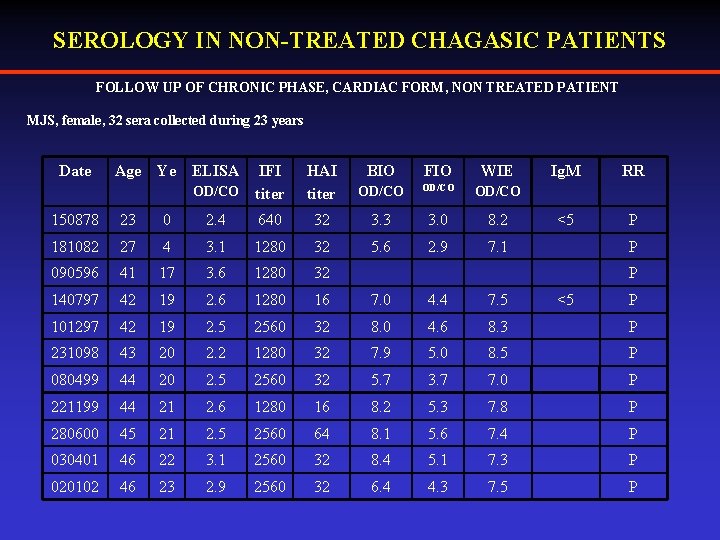
SEROLOGY IN NON-TREATED CHAGASIC PATIENTS FOLLOW UP OF CHRONIC PHASE, CARDIAC FORM, NON TREATED PATIENT MJS, female, 32 sera collected during 23 years Date Age Ye ELISA HAI titer BIO FIO WIE OD/CO IFI titer OD/CO Ig. M RR <5 P 150878 23 0 2. 4 640 32 3. 3 3. 0 8. 2 181082 27 4 3. 1 1280 32 5. 6 2. 9 7. 1 090596 41 17 3. 6 1280 32 140797 42 19 2. 6 1280 16 7. 0 4. 4 7. 5 101297 42 19 2. 5 2560 32 8. 0 4. 6 8. 3 P 231098 43 20 2. 2 1280 32 7. 9 5. 0 8. 5 P 080499 44 20 2. 5 2560 32 5. 7 3. 7 7. 0 P 221199 44 21 2. 6 1280 16 8. 2 5. 3 7. 8 P 280600 45 21 2. 5 2560 64 8. 1 5. 6 7. 4 P 030401 46 22 3. 1 2560 32 8. 4 5. 1 7. 3 P 020102 46 23 2. 9 2560 32 6. 4 4. 3 7. 5 P P P <5 P

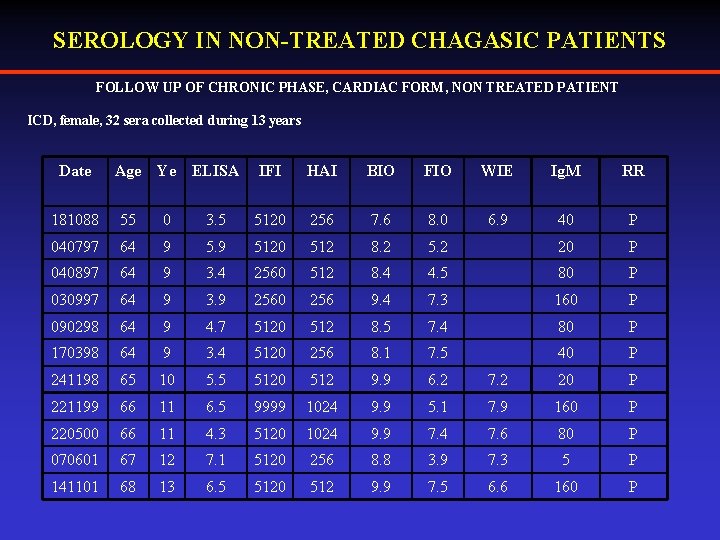
SEROLOGY IN NON-TREATED CHAGASIC PATIENTS FOLLOW UP OF CHRONIC PHASE, CARDIAC FORM, NON TREATED PATIENT ICD, female, 32 sera collected during 13 years Date Age Ye ELISA IFI HAI BIO FIO WIE Ig. M RR 6. 9 40 P 181088 55 0 3. 5 5120 256 7. 6 8. 0 040797 64 9 5120 512 8. 2 5. 2 20 P 040897 64 9 3. 4 2560 512 8. 4 4. 5 80 P 030997 64 9 3. 9 2560 256 9. 4 7. 3 160 P 090298 64 9 4. 7 5120 512 8. 5 7. 4 80 P 170398 64 9 3. 4 5120 256 8. 1 7. 5 40 P 241198 65 10 5. 5 5120 512 9. 9 6. 2 7. 2 20 P 221199 66 11 6. 5 9999 1024 9. 9 5. 1 7. 9 160 P 220500 66 11 4. 3 5120 1024 9. 9 7. 4 7. 6 80 P 070601 67 12 7. 1 5120 256 8. 8 3. 9 7. 3 5 P 141101 68 13 6. 5 5120 512 9. 9 7. 5 6. 6 160 P

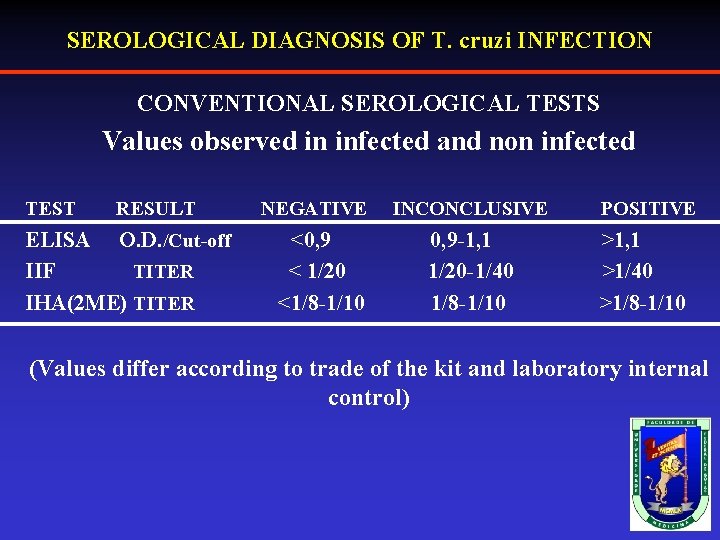
SEROLOGICAL DIAGNOSIS OF T. cruzi INFECTION CONVENTIONAL SEROLOGICAL TESTS Values observed in infected and non infected TEST RESULT ELISA O. D. /Cut-off IIF TITER IHA(2 ME) TITER NEGATIVE <0, 9 < 1/20 <1/8 -1/10 INCONCLUSIVE 0, 9 -1, 1 1/20 -1/40 1/8 -1/10 POSITIVE >1, 1 >1/40 >1/8 -1/10 (Values differ according to trade of the kit and laboratory internal control)

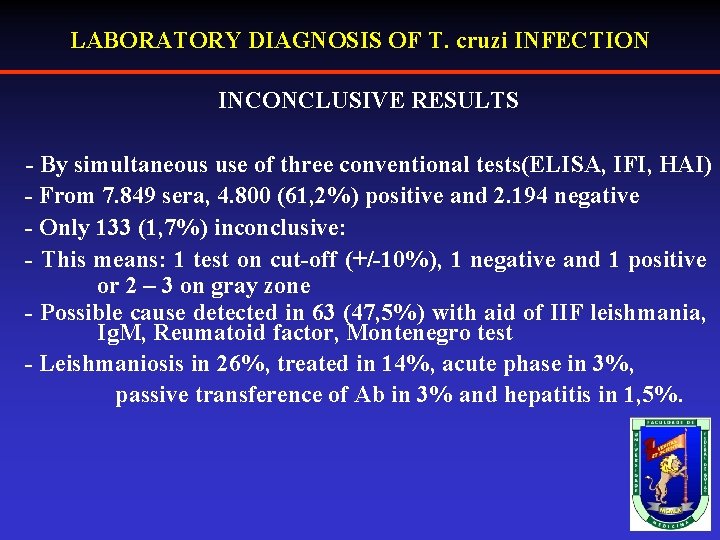
LABORATORY DIAGNOSIS OF T. cruzi INFECTION INCONCLUSIVE RESULTS - By simultaneous use of three conventional tests(ELISA, IFI, HAI) - From 7. 849 sera, 4. 800 (61, 2%) positive and 2. 194 negative - Only 133 (1, 7%) inconclusive: - This means: 1 test on cut-off (+/-10%), 1 negative and 1 positive or 2 – 3 on gray zone - Possible cause detected in 63 (47, 5%) with aid of IIF leishmania, Ig. M, Reumatoid factor, Montenegro test - Leishmaniosis in 26%, treated in 14%, acute phase in 3%, passive transference of Ab in 3% and hepatitis in 1, 5%.

LABORATOY DIAGNOSIS OF T. cruzi INFECTION SOME RESULTS OBTAINED IN NOT CLEARLY DEFINED SERA (n = 205/16. 150 sera), WITH NEW TOOLS - Agreement among recombinant antigens (at least three kits): All negative: 35 sera (11/35 do not agree with 4# ELISA) All positive: 61 sera (14 do not agree with 4# ELISA) -----Agreements: 96/205 (47 %) - Lack of agreement (some P, other N): 64 sera - Partial agreement (majority P or N): 45 sera - Those 109 sera, are P or N? What to do? Believe in which?

LABORATOY DIAGNOSIS OF T. cruzi INFECTION • RELIABLE RESULTS IN SEROLOGY – Matherials: approved kit, retested for lot at the lab (internal panel with low positives and high negatives) • Good laboratory practices: temperatures, p. H, etc. • POP : description of each procedure, in detail – Methods: Programs of technical training (Telelab) – Quality: Lab ought to participate in an External Quality Control Programme, provided that: • This programm send at least 2 panels/year • The Lab should be approved

LABORATOY DIAGNOSIS OF T. cruzi INFECTION • PROGRAMS of EXTERNAL QUALITY CONTROL – Initial difficulty to mount serum panels (AR/CH/BR/PY) Meeting OPS-BH 1994) – Difficult to obtain panels in non endemic countries – Initiative of OPS/PANEL São Paulo (BR) 1995 – Programm operative in >18 countries – Priority in reccommendations of South Cone (1999) – Programms of Hemotherapy Societies (AR, BR) – Programm of MPH, BR, COSAH>ANVISA (2001)

LABORATOY DIAGNOSIS OF T. cruzi INFECTION • CONTROL QUALITY PROGRAM OF THE MINISTRY OF HEALTH, BR FOR EXTERNAL CONTROL IN BLOOD BANKS – – – – – Joint venture National Agency of Sanitary Surveill. /Fiocruz Both belong to Ministery of Health, coordination, execution Technical Committee, 1 by area (syphilis, HIV, etc) Several meetings/year, evaluation of results by marker Three panels/year (6 x 3) – by post Started on 2000, processed 5. 718 plasma bags (2007) From 2001 to 2008, 18 evaluations, of 135 services (90% public) Increase in results, from 3, 6 % to 0, 9% discordances. ) Indirectly detected problems with different lots of used kits

LABORATOY DIAGNOSIS OF T. cruzi INFECTION • EVALUATION OF ELISA KITS AVAILABLE IN BRAZIL – Study performed by Lab Coordination, Min. Health – Bought all certified kits available in Brazil (n=12) – Selection of 152 sera (half negative) – Blind tests by 4 labs: MG, PE, MS, GO – Kappa index of 0, 71 to 0, 98. Sensitivity 0, 97 a 1, 0 – 6 kits sensitivity = 1, 0. 5 kits = 0, 99. 1 kit =0, 97. – Trades: Adaltis, Bioma, Biomerieux, Bioschil, Biozima, – Ebram, Hemagen, Omega, REM, Wama, Wiener – This study allows to exclude some trades, based on published data (available at the site of the M. Health)

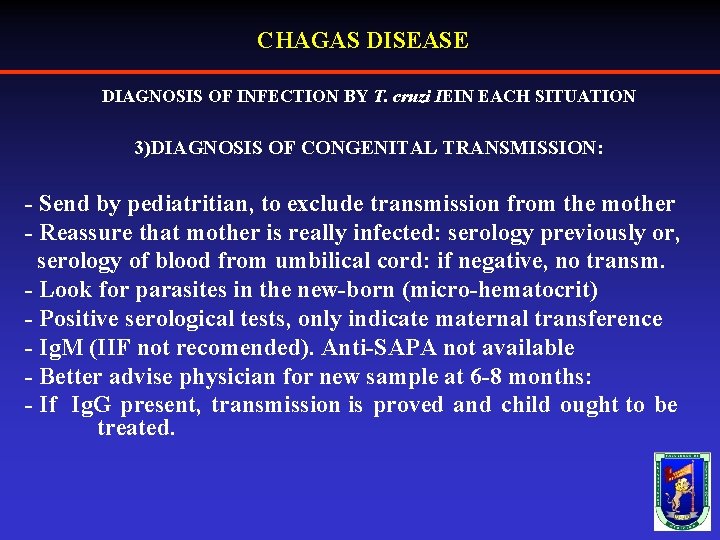
CHAGAS DISEASE DIAGNOSIS OF INFECTION BY T. cruzi IEIN EACH SITUATION 3)DIAGNOSIS OF CONGENITAL TRANSMISSION: - Send by pediatritian, to exclude transmission from the mother - Reassure that mother is really infected: serology previously or, serology of blood from umbilical cord: if negative, no transm. - Look for parasites in the new-born (micro-hematocrit) - Positive serological tests, only indicate maternal transference - Ig. M (IIF not recomended). Anti-SAPA not available - Better advise physician for new sample at 6 -8 months: - If Ig. G present, transmission is proved and child ought to be treated.

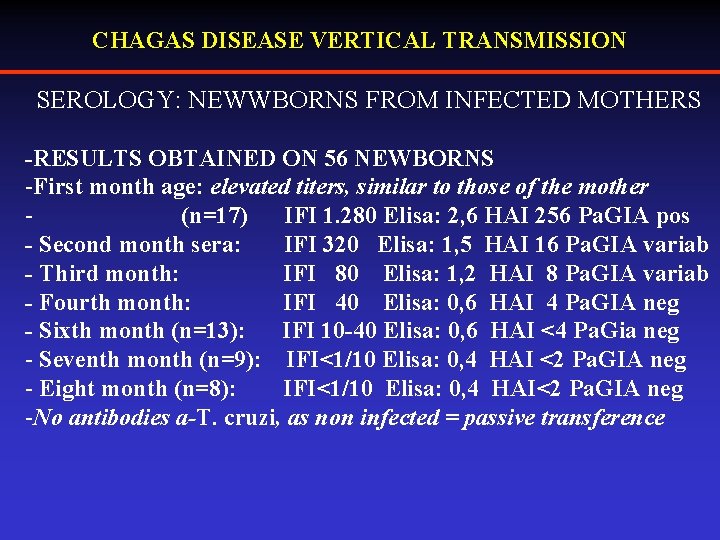
CHAGAS DISEASE VERTICAL TRANSMISSION SEROLOGY: NEWWBORNS FROM INFECTED MOTHERS -RESULTS OBTAINED ON 56 NEWBORNS -First month age: elevated titers, similar to those of the mother (n=17) IFI 1. 280 Elisa: 2, 6 HAI 256 Pa. GIA pos - Second month sera: IFI 320 Elisa: 1, 5 HAI 16 Pa. GIA variab - Third month: IFI 80 Elisa: 1, 2 HAI 8 Pa. GIA variab - Fourth month: IFI 40 Elisa: 0, 6 HAI 4 Pa. GIA neg - Sixth month (n=13): IFI 10 -40 Elisa: 0, 6 HAI <4 Pa. Gia neg - Seventh month (n=9): IFI<1/10 Elisa: 0, 4 HAI <2 Pa. GIA neg - Eight month (n=8): IFI<1/10 Elisa: 0, 4 HAI<2 Pa. GIA neg -No antibodies a-T. cruzi, as non infected = passive transference

CHAGAS DISEASE DIAGNOSIS OF INFECTION BY T. cruzi IN EACH CONTEXT 4) FOLLOW UP OF A SPECIFICALLY TREATED: -Send by physician, looking for cure --For Tc 2 b: - Cure is possible in all newborns, 60% of acute phase and children and up to 25% of adults, after benznidazol x 60 days - Cure criteria is abscence of antibodies, formerly present - Time to attain: months in congenital, years in acute/children and decades in adults - For these, need to preserve previous sera with glicerol - To procede in paralel with sera before and after treatment

SEROLOGY IN TREATED CHAGASIC PATIENTS FOLLOW UP OF ACUTE PHASE (50 DAYS EVOLUTION) IN A SUCCESFULLY TREATED PATIENT DPS, female, fever by 07/03/81, Romaña sign. Flagellates on wet smear. Benznidazol x 60 days. Date Age TT ELISA IFI HAI BIO FIO WIE Ig. M RR 640 P 270481 10 0 1. 4 160 64 1. 2 6. 2 7. 0 110581 10 15 1. 7 1280 32 3. 5 4. 2 7. 4 P 090981 10 5 m 1. 0 20 16 1. 9 3. 4 2. 3 N 280482 11 1 y 1. 2 10 4 1. 1 2. 3 0. 5 N 221183 12 2 y 1. 2 10 <2 1. 4 4. 1 0. 1 N 090584 13 3 y 0. 9 10 4 1. 2 1. 8 0. 1 N 250785 14 4 y 0. 9 <10 <4 0. 9 1. 1 0. 1 N 310787 16 6 y 0. 8 <10 4 1. 0 1. 2 0. 3 N 280590 19 9 y 1. 1 <10 <4 0. 8 1. 1 0. 4 N 190494 22 13 0. 8 <10 4 0. 7 0. 5 0. 1 N 070596 25 15 0. 9 20 4 0. 6 0. 1 N

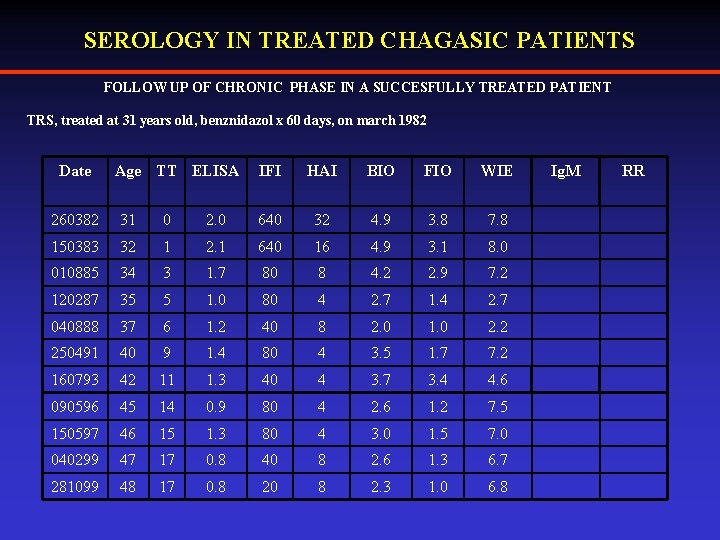
SEROLOGY IN TREATED CHAGASIC PATIENTS FOLLOW UP OF CHRONIC PHASE IN A SUCCESFULLY TREATED PATIENT TRS, treated at 31 years old, benznidazol x 60 days, on march 1982 Date Age TT ELISA IFI HAI BIO FIO WIE 260382 31 0 2. 0 640 32 4. 9 3. 8 7. 8 150383 32 1 2. 1 640 16 4. 9 3. 1 8. 0 010885 34 3 1. 7 80 8 4. 2 2. 9 7. 2 120287 35 5 1. 0 80 4 2. 7 1. 4 2. 7 040888 37 6 1. 2 40 8 2. 0 1. 0 2. 2 250491 40 9 1. 4 80 4 3. 5 1. 7 7. 2 160793 42 11 1. 3 40 4 3. 7 3. 4 4. 6 090596 45 14 0. 9 80 4 2. 6 1. 2 7. 5 150597 46 15 1. 3 80 4 3. 0 1. 5 7. 0 040299 47 17 0. 8 40 8 2. 6 1. 3 6. 7 281099 48 17 0. 8 20 8 2. 3 1. 0 6. 8 Ig. M RR

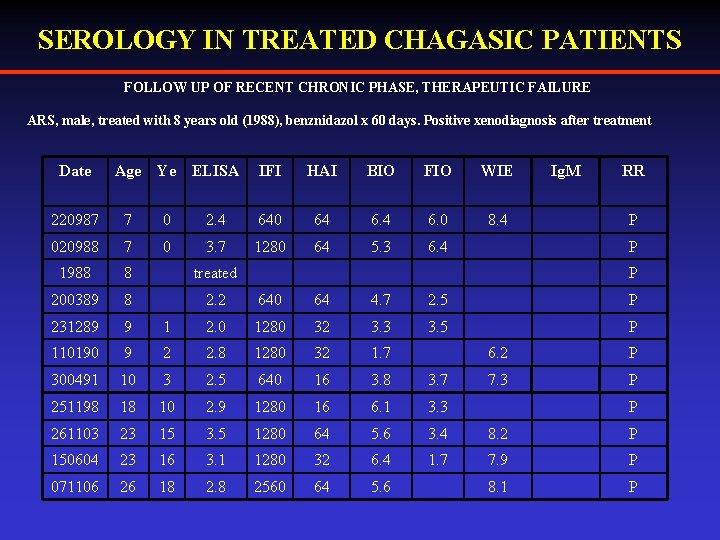
SEROLOGY IN TREATED CHAGASIC PATIENTS FOLLOW UP OF RECENT CHRONIC PHASE, THERAPEUTIC FAILURE ARS, male, treated with 8 years old (1988), benznidazol x 60 days. Positive xenodiagnosis after treatment Date Age Ye ELISA IFI HAI BIO FIO WIE 8. 4 Ig. M RR 220987 7 0 2. 4 640 64 6. 0 P 020988 7 0 3. 7 1280 64 5. 3 6. 4 1988 8 treated 200389 8 2. 2 640 64 4. 7 2. 5 P 231289 9 1 2. 0 1280 32 3. 3 3. 5 P 110190 9 2 2. 8 1280 32 1. 7 300491 10 3 2. 5 640 16 3. 8 3. 7 251198 18 10 2. 9 1280 16 6. 1 3. 3 261103 23 15 3. 5 1280 64 5. 6 3. 4 8. 2 P 150604 23 16 3. 1 1280 32 6. 4 1. 7 7. 9 P 071106 26 18 2. 8 2560 64 5. 6 8. 1 P P P 6. 2 P 7. 3 P P

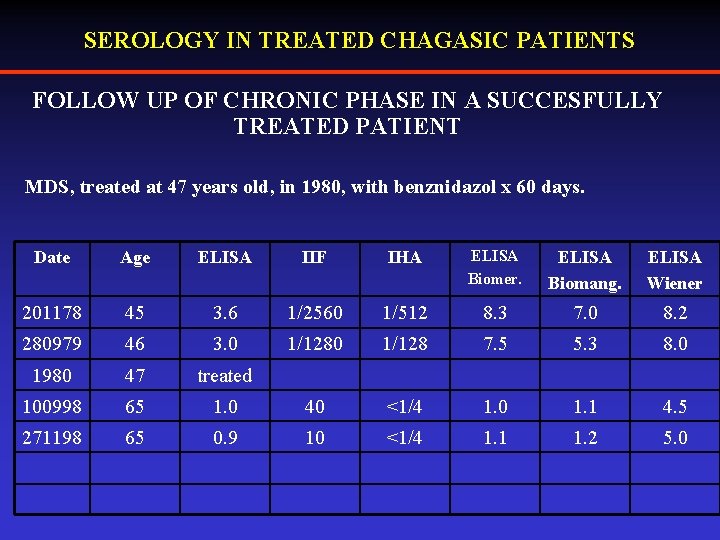
SEROLOGY IN TREATED CHAGASIC PATIENTS FOLLOW UP OF CHRONIC PHASE IN A SUCCESFULLY TREATED PATIENT MDS, treated at 47 years old, in 1980, with benznidazol x 60 days. Date Age ELISA IIF IHA ELISA Biomer. ELISA Biomang. ELISA Wiener 201178 45 3. 6 1/2560 1/512 8. 3 7. 0 8. 2 280979 46 3. 0 1/128 7. 5 5. 3 8. 0 1980 47 treated 100998 65 1. 0 40 <1/4 1. 0 1. 1 4. 5 271198 65 0. 9 10 <1/4 1. 1 1. 2 5. 0

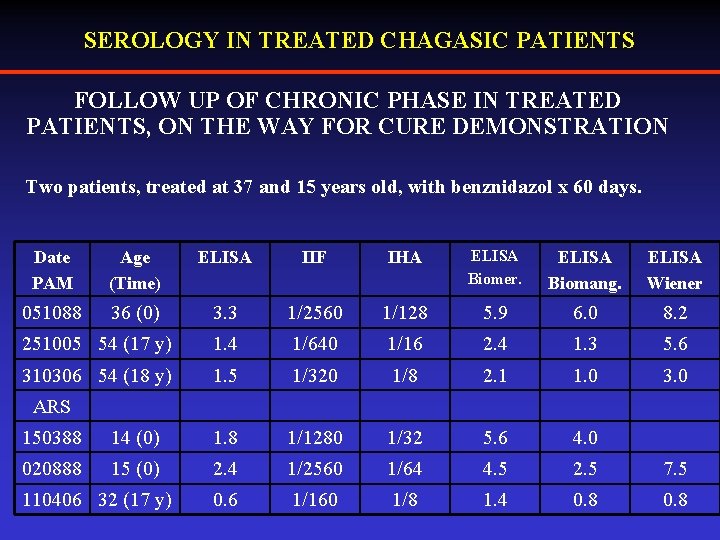
SEROLOGY IN TREATED CHAGASIC PATIENTS FOLLOW UP OF CHRONIC PHASE IN TREATED PATIENTS, ON THE WAY FOR CURE DEMONSTRATION Two patients, treated at 37 and 15 years old, with benznidazol x 60 days. Date PAM Age (Time) ELISA IIF IHA ELISA Biomer. ELISA Biomang. ELISA Wiener 051088 36 (0) 3. 3 1/2560 1/128 5. 9 6. 0 8. 2 251005 54 (17 y) 1. 4 1/640 1/16 2. 4 1. 3 5. 6 310306 54 (18 y) 1. 5 1/320 1/8 2. 1 1. 0 3. 0 ARS 150388 14 (0) 1. 8 1/1280 1/32 5. 6 4. 0 020888 15 (0) 2. 4 1/2560 1/64 4. 5 2. 5 7. 5 0. 6 1/160 1/8 1. 4 0. 8 110406 32 (17 y)

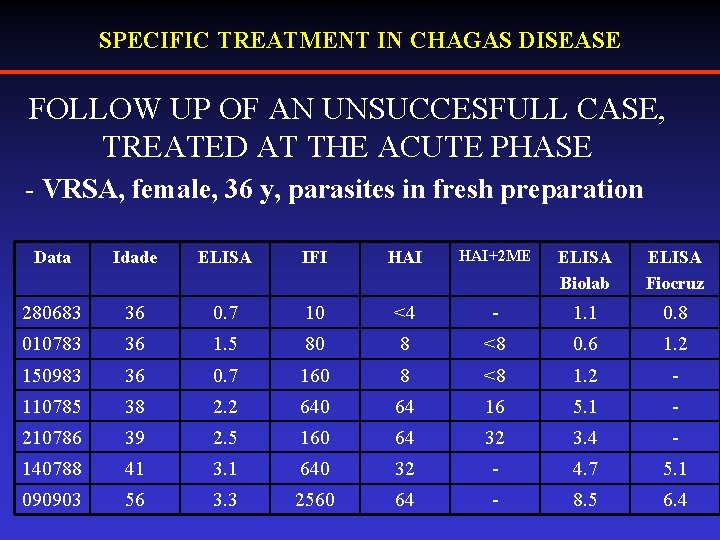
SPECIFIC TREATMENT IN CHAGAS DISEASE FOLLOW UP OF AN UNSUCCESFULL CASE, TREATED AT THE ACUTE PHASE - VRSA, female, 36 y, parasites in fresh preparation Data Idade ELISA IFI HAI+2 ME ELISA Biolab ELISA Fiocruz 280683 36 0. 7 10 <4 - 1. 1 0. 8 010783 36 1. 5 80 8 <8 0. 6 1. 2 150983 36 0. 7 160 8 <8 1. 2 - 110785 38 2. 2 640 64 16 5. 1 - 210786 39 2. 5 160 64 32 3. 4 - 140788 41 3. 1 640 32 - 4. 7 5. 1 090903 56 3. 3 2560 64 - 8. 5 6. 4

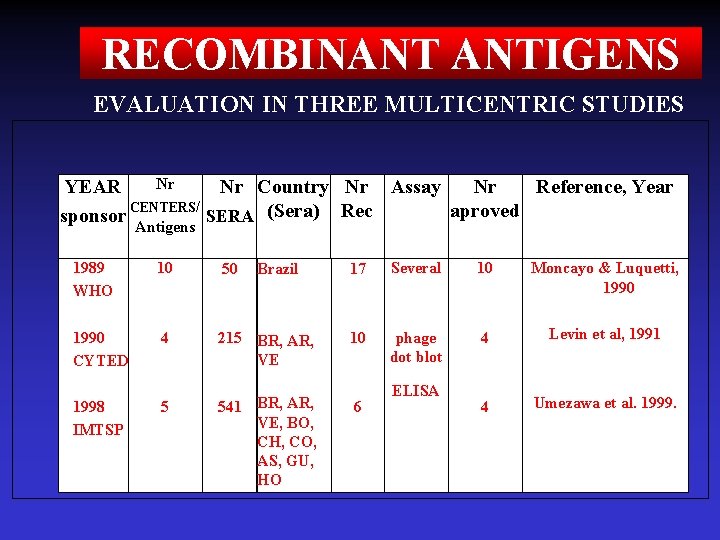
RECOMBINANT ANTIGENS EVALUATION IN THREE MULTICENTRIC STUDIES Nr YEAR Nr Country Nr Assay Nr Reference, Year / aproved sponsor CENTERS SERA (Sera) Rec Antigens 1989 WHO 10 50 1990 CYTED 4 215 BR, AR, VE 1998 IMTSP 5 541 BR, AR, VE, BO, CH, CO, AS, GU, HO Brazil 17 Several 10 Moncayo & Luquetti, 1990 10 phage dot blot 4 Levin et al, 1991 4 Umezawa et al. 1999. 6 ELISA

DIAGNOSIS & TREATMENT OF T. cruzi INFECTION SOME OF THE GREAT DREAMS that latinamericans transformed in facts 1)CONSENSUS ON THE BETTER NEW REAGENTS FOR SEROLOGICAL DIAGNOSIS: multicentric study WHO 19891990, Moncayo & Luquetti, 1990. 2)ELIMINATION OF TRANSMISSION BY Triatoma infestans AT THE SOUTH CONE, 1991: 3 certified countries(UR, CH, BR)and 2 in advanced stage (PY, BO) 3)CONSENSUS Tc 1/Tc 2, Rio, 1999. 4)CONSENSUS ON PCR PROCEDURES AND OPTIMIZATION OF SPECIFICITY, TDR, Buenos Aires, 2008 5)WHO reference Serum: Prototypes Tc 1 & Tc 2 lyophilization (2 L) scheduled, 2009.

DIAGNOSIS & TREATMENT OF T. cruzi INFECTION CONCLUSION 1) Most diagnostic situations are easily solved by serology at the chronic phase 2) Serological diagnosis nowadays is precise and reliable 3) Cure is return to non infected status: i. e. no parasites, nor antibodies (Cancado) 3) In succesfully treated patients, serological tests became negative after a period of time 4) It is expected to have same curve than passive transmission: no Ag, Ab should come down 5) Immunological memory, made the difference: parasites by weeks (acute), Ab dissapear in months. parasites < 10 y (recent chronic): Ab clear in few years parasites > 10 y: Ab start to decline after 2 decades, negative after 3 -4 decades 6) Individual differences. Recombinants not better than crude. Purified good for individual cases. Single tool not enough.

COLLABORATORS / PARTNERS OF RESEARCH, CHAGAS DISEASE Goiânia: Dr. Joffre M. de Rezende (Gastroenterology) Dr. Anis Rassi (Cardiology) Dr. Helio Moreira (Proctology) Dr. Ênio Chaves de Oliveira (Surgery esophagus and colon) Dra. Dayse Elizabeth Campos (Cardiology) Dra. Maria da Glória Merheb Vaz (Gastroenterology) Dra. Rita Francis G. y R. Branco (Cardiopediatry) Dra. Neusa G. Leal Marra (Cardiology in obstetrics) Psic. Auta Mendes (Psicology) Dr. Ionizete Garcia da Silva (xenodiagnosis) (IPTSP, UFG) Dra. Ana Maria de Castro (hemoculture) (IPTSP, UFG) São Paulo Dra. Nobuko Yoshida (Unifesp) Dr. José Franco da Silveira (Unifesp) Dra. Eufrozina S. Umezawa (USP) Rio Jan. Dr. Octávio Fernandes (Fiocruz) Uberaba Dr. Aluisio R. Prata (FM Triângulo Mineiro) Argentina Dr. Mariano Levin and Alejandro Schijman (markers, PCR) U. S. A. Dr. Miercio Pereira (markers) U. K. Dr. Michael A. Miles (strains)
 A subsequent
A subsequent Serological testing software
Serological testing software Dr mohit bhatia varanasi
Dr mohit bhatia varanasi Chapter 35 immune system and disease
Chapter 35 immune system and disease Chagas
Chagas Sintomas fase aguda doença de chagas
Sintomas fase aguda doença de chagas Disenteria amebiana
Disenteria amebiana Vetor chagas
Vetor chagas Vetor chagas
Vetor chagas Instituto evandro chagas
Instituto evandro chagas Metabolic action of growth hormone
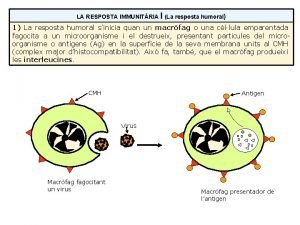
Metabolic action of growth hormone The difference between humoral and cell mediated immunity
The difference between humoral and cell mediated immunity Humoral stimulus
Humoral stimulus Thymosin and thymopoietin assist in the maturation of:
Thymosin and thymopoietin assist in the maturation of: Effector mechanism of humoral immunity
Effector mechanism of humoral immunity Celular
Celular Reactantes de fase aguda
Reactantes de fase aguda Pmn
Pmn Humoral patoloji paradigması
Humoral patoloji paradigması Limfoblast
Limfoblast Inmunidad adaptativa
Inmunidad adaptativa Humoral immunity
Humoral immunity Explain nursing process
Explain nursing process Medical diagnosis and nursing diagnosis difference
Medical diagnosis and nursing diagnosis difference Medical diagnosis and nursing diagnosis difference
Medical diagnosis and nursing diagnosis difference Types of nursing diagnoses
Types of nursing diagnoses Communicable disease and non communicable disease
Communicable disease and non communicable disease Perbedaan diagnosis gizi dan diagnosis medis
Perbedaan diagnosis gizi dan diagnosis medis Nursing diagnosis for vision impairment
Nursing diagnosis for vision impairment Nursing diagnosis for meniere's disease
Nursing diagnosis for meniere's disease