International Symposium Centenary of Chagas Disease 1909 2009




- Slides: 17

International Symposium Centenary of Chagas Disease 1909. 2009 July, 2009 – Rio de Janeiro Use of Polymerase Chain Reaction (PCR) to Establish Drug Efficacy - Value and Limitations Constança Britto cbritto@ioc. fiocruz. br Oswaldo Cruz Foundation, FIOCRUZ

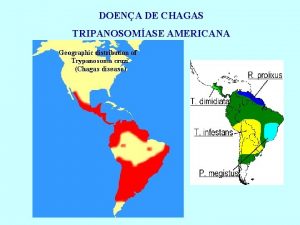
I- Etiological Treatment should focus on Parasite Clearance Chemotherapy during the acute stage of Chagas disease leads to parasitological cure in up to 80% of the cases, but its effectiveness in the long-lasting chronic phase remains unclear. The main limitations are: (i) long-term follow-up (persistence of specific antibodies); (ii) continuing administration of toxic nitroimidazole derivatives; (iii) differences in drug susceptibility among distinct T. cruzi strains; (iv) lack of reliable tests to ensure parasite elimination. Ø Conventional Serology may remain positive for several years despite repeated negative parasite detection methods. Ø Rather poor sensitivity of Xenodiagnosis and Hemocultive

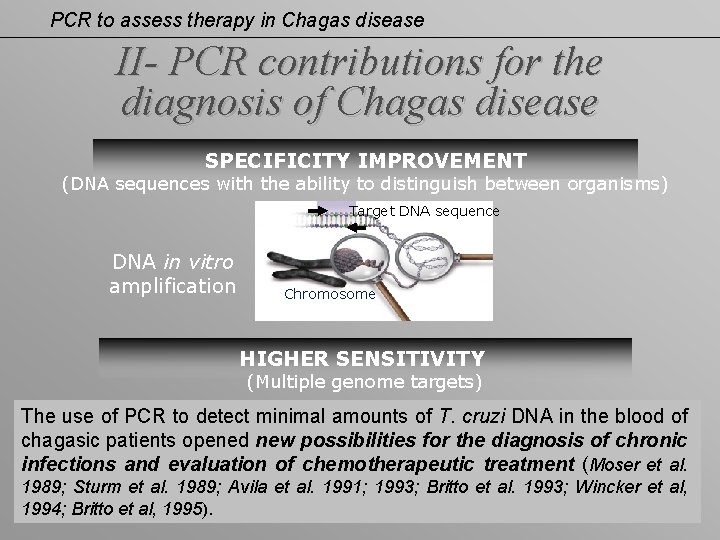
PCR to assess therapy in Chagas disease II- PCR contributions for the diagnosis of Chagas disease SPECIFICITY IMPROVEMENT (DNA sequences with the ability to distinguish between organisms) Target DNA sequence DNA in vitro amplification Chromosome HIGHER SENSITIVITY (Multiple genome targets) The use of PCR to detect minimal amounts of T. cruzi DNA in the blood of chagasic patients opened new possibilities for the diagnosis of chronic infections and evaluation of chemotherapeutic treatment (Moser et al. 1989; Sturm et al. 1989; Avila et al. 1991; 1993; Britto et al. 1993; Wincker et al, 1994; Britto et al, 1995).

III- Is PCR a valid technique to address cure? PCR performance for the analysis of clinical specimens has revealed discordant results concerning sensitivity, depending on: (i) epidemiological characteristics of the studied populations (geographic areas, high genetic heterogeneity of T. cruzi strains, etc); (ii) volume of blood collected (intermittent presence and quantity of circulating parasites at the time of blood collection); (iii) method used to isolate DNA; (iv) selection of parasite target-sequences, reagents used, as well as thermo-cycling conditions. Several therapeutic studies confirm the usefulness of PCR to evaluate treatment outcome in Chagas disease, as a complement to serological tests.

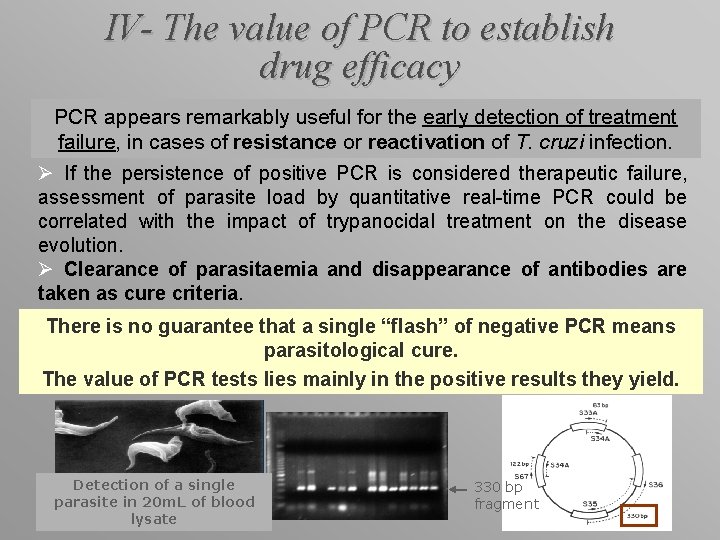
IV- The value of PCR to establish drug efficacy PCR appears remarkably useful for the early detection of treatment failure, in cases of resistance or reactivation of T. cruzi infection. Ø If the persistence of positive PCR is considered therapeutic failure, assessment of parasite load by quantitative real-time PCR could be correlated with the impact of trypanocidal treatment on the disease evolution. Ø Clearance of parasitaemia and disappearance of antibodies are taken as cure criteria. There is no guarantee that a single “flash” of negative PCR means parasitological cure. The value of PCR tests lies mainly in the positive results they yield. Detection of a single parasite in 20 m. L of blood lysate 330 bp fragment

V- Treatment of congenital infections Ø Parasite search by microhematocrit, hemoculture and PCR-based on satellite nuclear DNA (100 µL plasma) Ø PCR (during treatment & at the end of follow-up) confirmed 100% of Bz treatment efficacy in association with conventional serology negativation (Russomando et al, 1998). Parasite clearance more rapidly assessed by PCR [15 days after treatment] than serology [3 -8 months] during a 4 years follow-up (Russomando et al, 1998). Ø Schijman et al (2003) evaluated treatment outcome (Nf and Bz) in 40 infected infants monitored for 2 -3 years by PCR (satellite DNA) and conventional methods. Two m. L of blood were collected for PCR. Cure achieved by negativation of PCR + serology in 100% of infants with <3 months, in 66. 7% between 7 months-2 years old and in 12. 5% with >3 years old. In those infants who started therapy in their first months of life (<3 months), cure was achieved early after treatment.

VI- Treatment in early life Infants infected during the early years of life represent those patients amenable to specific treatment with great possibility of cure (Andrade et al. 1996; Sosa Estani et al. 1998). Galvão et al, 2003: J Clin Microbiol 41: 5066 -70. Ø Cohort of T. cruzi seropositive schoolchildren from an endemic Brazilian area; Ø Blood samples (5 m. L) were taken at baseline and 36 months after treatment with Bz (n=58) or placebo (n=53); Ø Bz group: 39. 6% positive PCR (for k. DNA) Placebo: 64. 2% positive PCR (P=0. 01) Ø Untreated patients had 1. 6 -fold-higher chance of remaining PCR positive than the Bz group (P<0. 05); Ø Adequate correlation between high proportion of negative PCR and decrease in antibody titers in the Bz group; Ø PCR positivity occurred in patients without reductions in antibody titers; Ø PCR is a useful tool for revealing therapeutic failure on a short-term basis.

VII- Treatment of chronic infections The efficacy of available drugs is limited for chronic patients (Urbina, 2002). The drugs are able to reduce the parasite population in blood and the risk for disease progression (Viotti et al, 1994; 2006). Up to 80% of chronic treated patients do not achieve parasitological cure as assessed by the persistence of positive serology (very slow decrease in antibody titers), now confirmed using PCR-based methods. (Britto C et al, 1995) Polymerase chain reaction detection of Trypanosoma cruzi in human blood samples as a tool for diagnosis and treatment evaluation. Parasitology 110: 241 -247. 32 Bz-treated seropositive chronic patients attending the Hospital of Infectious Diseases (Fiocruz). Positive PCR (k. DNA) observed in only 9 out of 32 treated patients who remained reactive with conventional serology (5 years post-treatment).

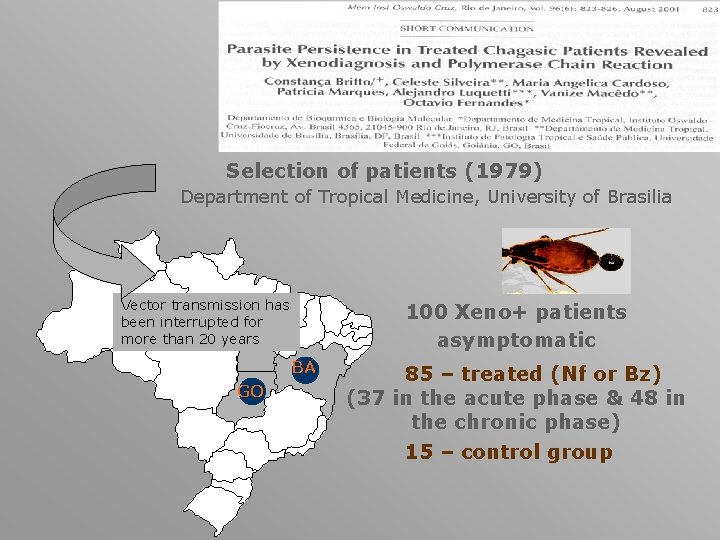
Selection of patients (1979) Department of Tropical Medicine, University of Brasilia Vector transmission has been interrupted for more than 20 years 100 Xeno+ patients asymptomatic BA GO 85 – treated (Nf or Bz) (37 in the acute phase & 48 in the chronic phase) 15 – control group

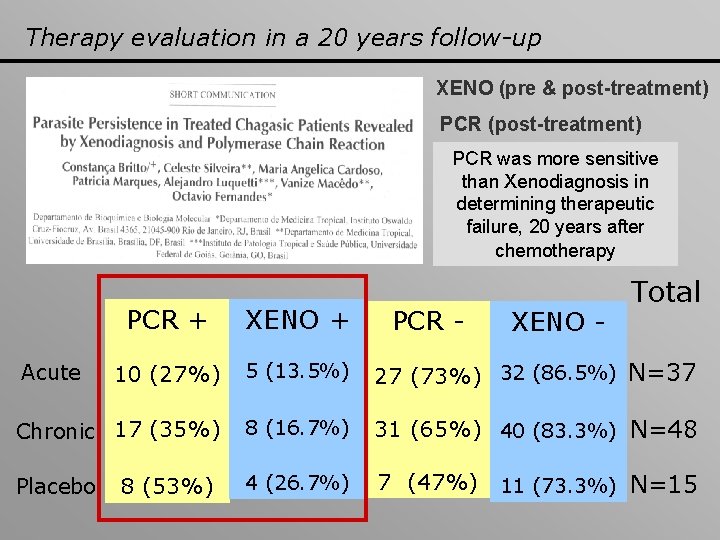
Therapy evaluation in a 20 years follow-up XENO (pre & post-treatment) PCR (post-treatment) PCR was more sensitive than Xenodiagnosis in determining therapeutic failure, 20 years after chemotherapy Total PCR + XENO + 10 (27%) 5 (13. 5%) 27 (73%) 32 (86. 5%) N=37 Chronic 17 (35%) 8 (16. 7%) 31 (65%) 40 (83. 3%) N=48 4 (26. 7%) 7 (47%) 11 (73. 3%) N=15 Acute Placebo 8 (53%) PCR - XENO -

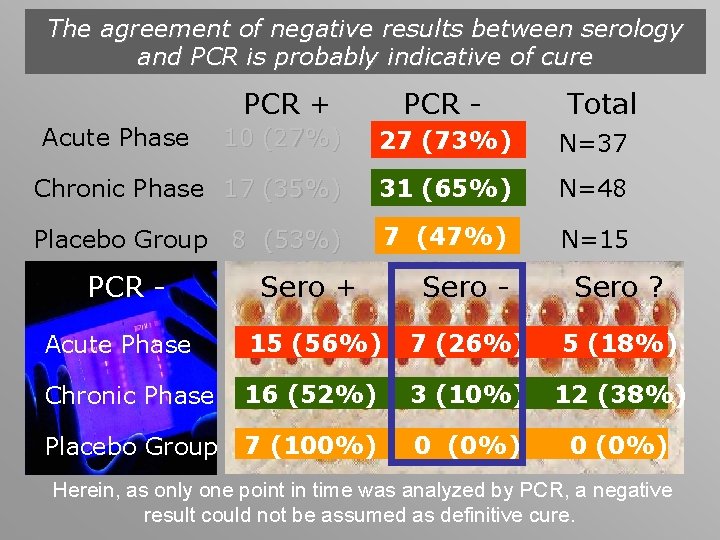
The agreement of negative results between serology and PCR is probably indicative of cure PCR + PCR - 10 (27%) 27 (73%) N=37 Chronic Phase 17 (35%) 31 (65%) N=48 Placebo Group 8 (53%) 7 (47%) N=15 Acute Phase PCR - Total Sero + Sero - Sero ? Acute Phase 15 (56%) 7 (26%) 5 (18%) Chronic Phase 16 (52%) 3 (10%) 12 (38%) Placebo Group 7 (100%) 0 (0%) Herein, as only one point in time was analyzed by PCR, a negative result could not be assumed as definitive cure.

Treatment of patients with Chronic Chagas Cardiomyopathy (CCC) Multicenter, randomized, placebo controlled clinical trial of 3, 000 patients with CCC (excludes severe disease). Full-scale trial follow-up will be 5 years Am Heart J 2008; 156: 37 -43 (ending in 2011). BENEFIT includes a pilot study (600 patients) to evaluate whether 60 days of Benznidazole therapy will reduce parasite activity determined by PCR negativation and parasite load measure. PCR performed at randomization, at the end of therapy (60 days) and after 2 years of follow-up. Participation of 8 countries and 60 centers, conducted by the Canadian Institute of Health Research and WHO/TDR, with the Latin American coordinating center at Dante Pazzanese Institute (SP, Brazil). PCR Core Lab in Brazil (Oswaldo Cruz Institute/FIOCRUZ, RJ) = responsible for PCR analyses from 16 Brazilian centers. This is the largest trial yet conducted in Chagas disease. The study will clarify the role of trypanocidal therapy in preventing cardiac disease progression and death.

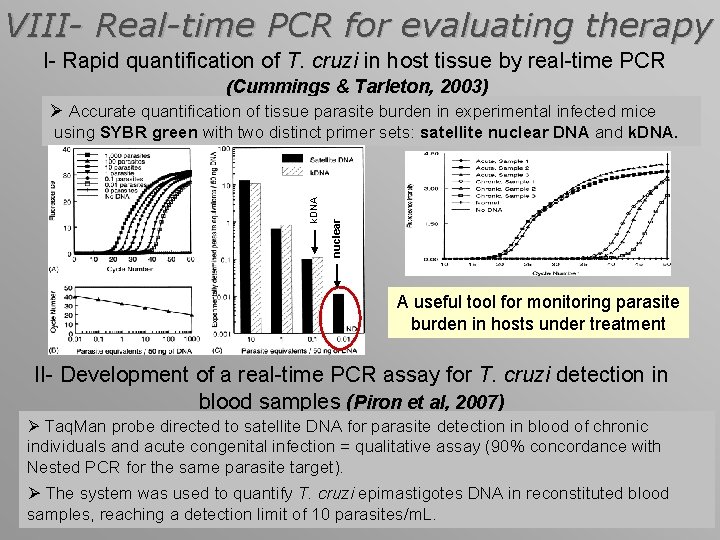
VIII- Real-time PCR for evaluating therapy I- Rapid quantification of T. cruzi in host tissue by real-time PCR (Cummings & Tarleton, 2003) Ø Accurate quantification of tissue parasite burden in experimental infected mice nuclear k. DNA using SYBR green with two distinct primer sets: satellite nuclear DNA and k. DNA. A useful tool for monitoring parasite burden in hosts under treatment II- Development of a real-time PCR assay for T. cruzi detection in blood samples (Piron et al, 2007) Ø Taq. Man probe directed to satellite DNA for parasite detection in blood of chronic individuals and acute congenital infection = qualitative assay (90% concordance with Nested PCR for the same parasite target). Ø The system was used to quantify T. cruzi epimastigotes DNA in reconstituted blood samples, reaching a detection limit of 10 parasites/m. L.

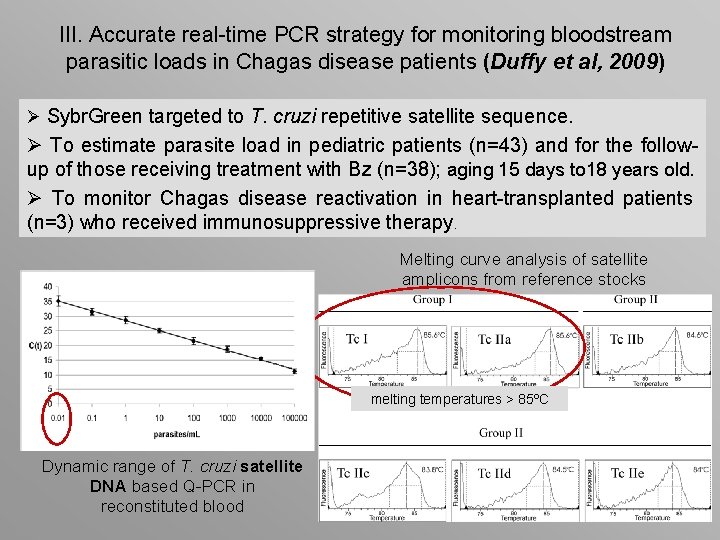
III. Accurate real-time PCR strategy for monitoring bloodstream parasitic loads in Chagas disease patients (Duffy et al, 2009) Ø Sybr. Green targeted to T. cruzi repetitive satellite sequence. Ø To estimate parasite load in pediatric patients (n=43) and for the followup of those receiving treatment with Bz (n=38); aging 15 days to 18 years old. Ø To monitor Chagas disease reactivation in heart-transplanted patients (n=3) who received immunosuppressive therapy. Melting curve analysis of satellite amplicons from reference stocks melting temperatures > 85ºC Dynamic range of T. cruzi satellite DNA based Q-PCR in reconstituted blood

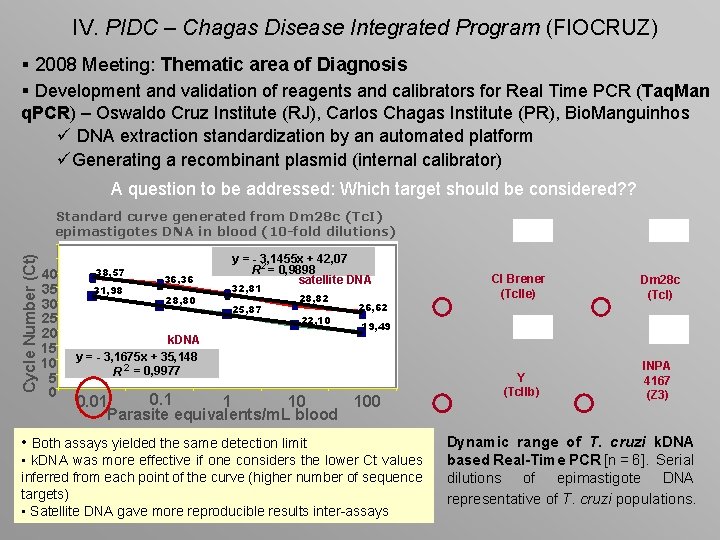
IV. PIDC – Chagas Disease Integrated Program (FIOCRUZ) § 2008 Meeting: Thematic area of Diagnosis § Development and validation of reagents and calibrators for Real Time PCR (Taq. Man q. PCR) – Oswaldo Cruz Institute (RJ), Carlos Chagas Institute (PR), Bio. Manguinhos ü DNA extraction standardization by an automated platform üGenerating a recombinant plasmid (internal calibrator) A question to be addressed: Which target should be considered? ? Cycle Number (Ct) Standard curve generated from Dm 28 c (Tc. I) epimastigotes DNA in blood (10 -fold dilutions) 40 35 30 25 20 15 10 5 0 38, 57 31, 98 36, 36 28, 80 y = - 3, 1455 x + 42, 07 R 2 = 0, 9898 satellite DNA 32, 81 25, 87 28, 82 22, 10 26, 62 Cl Brener (Tc. IIe) Dm 28 c (Tc. I) Y (Tc. IIb) INPA 4167 (Z 3) 19, 49 k. DNA y = - 3, 1675 x + 35, 148 R 2 = 0, 9977 0. 1 0. 01 1 10 100 Parasite equivalents/m. L blood • Both assays yielded the same detection limit • k. DNA was more effective if one considers the lower Ct values inferred from each point of the curve (higher number of sequence targets) • Satellite DNA gave more reproducible results inter-assays Dynamic range of T. cruzi k. DNA based Real-Time PCR [n = 6]. Serial dilutions of epimastigote DNA representative of T. cruzi populations.

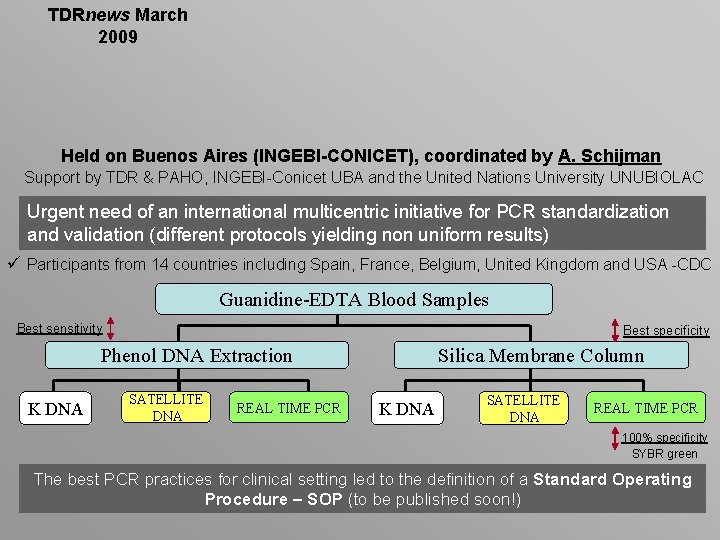
TDRnews March 2009 Held on Buenos Aires (INGEBI-CONICET), coordinated by A. Schijman Support by TDR & PAHO, INGEBI-Conicet UBA and the United Nations University UNUBIOLAC Urgent need of an international multicentric initiative for PCR standardization and validation (different protocols yielding non uniform results) ü Participants from 14 countries including Spain, France, Belgium, United Kingdom and USA -CDC Guanidine-EDTA Blood Samples Best sensitivity Best specificity Phenol DNA Extraction K DNA SATELLITE DNA REAL TIME PCR Silica Membrane Column K DNA SATELLITE DNA REAL TIME PCR 100% specificity SYBR green The best PCR practices for clinical setting led to the definition of a Standard Operating Procedure – SOP (to be published soon!)

THANK YOU. . . AND ENJOY RIO !!!!
 Sintomas fase aguda doença de chagas
Sintomas fase aguda doença de chagas Instituto evandro chagas
Instituto evandro chagas Sintomas fase aguda doença de chagas
Sintomas fase aguda doença de chagas Vetor chagas
Vetor chagas Chagas
Chagas Vetor chagas
Vetor chagas International perforating symposium
International perforating symposium International perforating symposium
International perforating symposium International perforating symposium
International perforating symposium International symposium on molecular spectroscopy
International symposium on molecular spectroscopy International police executive symposium
International police executive symposium International perforating symposium
International perforating symposium International perforating symposium
International perforating symposium International perforating symposium
International perforating symposium 1909
1909 Trait and factor theory steps
Trait and factor theory steps Antun gustav matoš kip domovine leta 188 analiza
Antun gustav matoš kip domovine leta 188 analiza Nata a torino nel 1909
Nata a torino nel 1909