EPIDEMIOLOGY CONTROL AND SURVEILLANCE OF CHAGAS DISEASE Jos













- Slides: 19

EPIDEMIOLOGY, CONTROL AND SURVEILLANCE OF CHAGAS DISEASE José Rodrigues Coura Laboratório de Doenças Parasitárias Medicina Tropical Instituto Oswaldo Cruz - Fiocruz Rio de Janeiro

INTRODUCTION • Since Carlos Chagas discovered American Trypanosomiasis in 1909, knowledge about the epidemiology of the disease has evolved in three welldefined phases: • The discovery phase • The phase of knowledge dissemination • The phase of knowledge application for its control and surveillance • Carlos Chagas discovered not only a new human disease. He discovery first a new parasite, its vector and domestic reservoir, the human disease itself and the wild cycle of the infection. This is unique in the medical and biological history


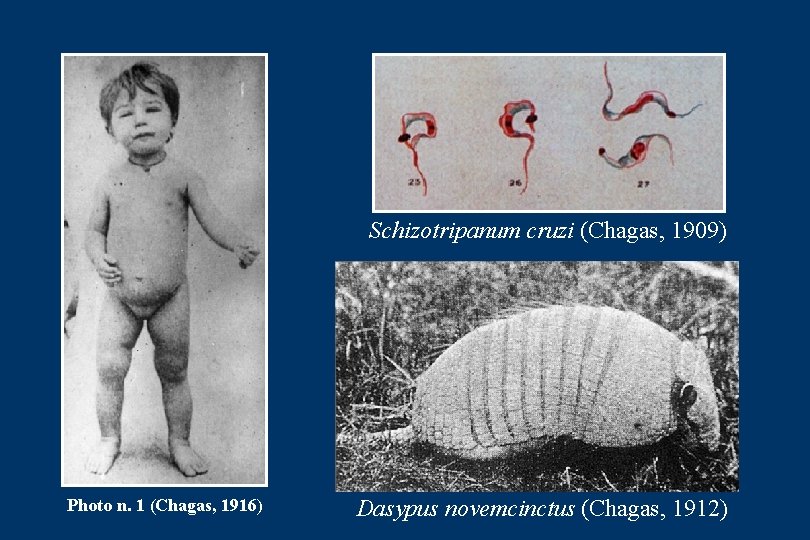
THE DISCOVERY PHASE • The discovery phase correspond to the pioneering studies by Carlos Chagas on Trypanosoma cruzi in Conorhinus megistus (Pantrongylus megistus) • The experimental infections in laboratory animals, mice, guinea-pigs and dogs, the fiding of a naturally infected cat with T. cruzi and the discription of the first acute cases of the disease (Chagas 1908, 1909) • In 1912, Chagas discovered that armadillo (Dasypus novemcinctus) was a wild reservoir of T. cruzi. Concomitantly, in the same ecotope, he found Triatoma geniculatus (Panstrongylus geniculatus) infected with the parasite, thereby defining the wild cycle of the Chagas disease • The clinical and pathogenesis descriptions of the acute form by Chagas (1916) along those of the chronic form by Chagas & Villela (1922) complemented most of the knowledge on the disease • This was subsequently complemented with the description of T. cruzi among naturally infected monkeys in the State of Pará (Chagas 1924)

Conorrhinus and Chritidias (Chagas, 1908 – 1909)

Schizotripanum cruzi (Chagas, 1909) Photo n. 1 (Chagas, 1916) Dasypus novemcinctus (Chagas, 1912)

OTHER STUDIES DURING THE DISCOVERY PHASE • Arthur Neiva (1910) highlighted the classification and biology of Conorhinus megistus • Gaspar Vianna (1911) and Magarinus Torres (1917) deepened the pathology studies of Chagas disease • Brumpt (1912, 1914) studied the biology of T. cruzi, in vectors, its penetration through the ocular mucosa and described the xenodiagnosis • Guerreiro & Machado (1913) developed the complement fixation test (Bordet & Gengou reation) • Evandro Chagas (1930, 1932) made new studies on the cardiac form, including eletrocardiographic studies • Emmanuel Dias (1933, 1934) studied T. cruzi in the vertebrates and invertebrates, completing the studies on the parasite, its vectors, reservois and human infection

THE KNOWLEDGE DISSEMINATION PHASE • Carlos Chagas publicised his discoveries in speeches in Brazil and abroad, and in various papers published in portuguese, spanish, english, french and german • Salvador Mazza expanded the knowledged on Chagas disease publishing many acute cases of Chagas disease in Argentina through the study group for regional pathology (MEPRA) • Romaña (1935) described the ophthalmic-ganglionic complex, called Romaña sign, the most visible sign for recognition of the acute phase of Chagas disease • The creation of the “Prophylaxis and Study Center of Chagas disease” from Oswaldo Cruz Institute in Bambuí and the Medical Schools of Ribeirão Preto, Goiânia, Uberaba and Uberlândida in the endemic triangle • The International Congress commemorating the 50 th aniversary of the discovery of Chagas disease at Oswaldo Cruz Institute (1959)

THE KNOWLEDGE APPLICATION TO EPIDEMIOLOGY, CONTROL AND SURVEILLANCE OF CHAGAS DISEASE • Emmanuel Dias, Dias & Pelegrino and Laranja et al (1945 -1956) developed fundamental studies on epidemiology, vector control and Chagas disease cardiomiopathy in Minas Gerais • Pedreira de Freitas, Barretto, Almeida, Köberle and Ramos et al (1946 -1957) disclosed new aspects of ecology and vector control, serologic diagnosis, chagasic cardiomiopathy and pathology of Chagas disease in Ribeirão Preto, São Paulo • Di Primio (1950 -1955) mapping out the triatomines in Rio Grande do Sul • Lucena and Lucena et al (1940 -1962) studied the vectors and the epidemiology of Chagas disease in Northeast of Brazil • Rassi and Rezende et al (1956 -1960) described the acute phase, cardiac and digestive forms of Chagas disease in Goiás • Andrade, Prata, Alencar and Samuel Pessoa et al (1956 -1960) studied and spread out the knowledge of pathology and epidemiology of Chagas disease from Bahia to Ceará, within Northeastern Brazil

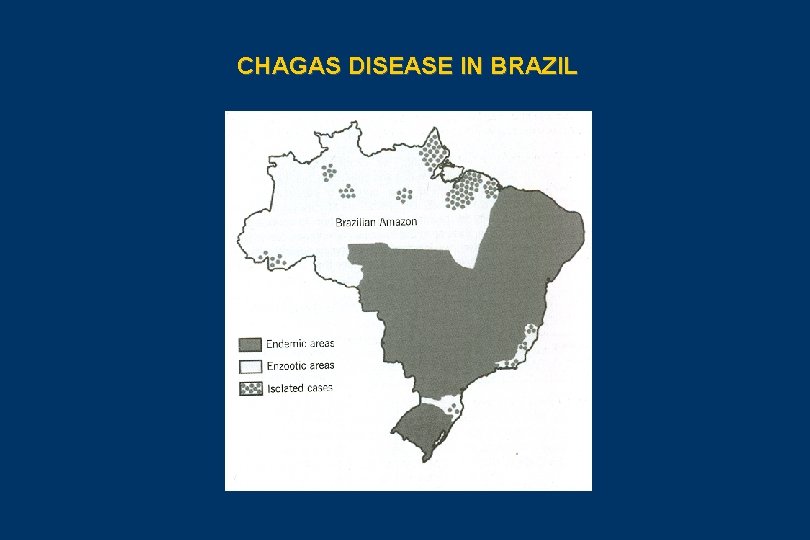
CHAGAS DISEASE IN BRAZIL

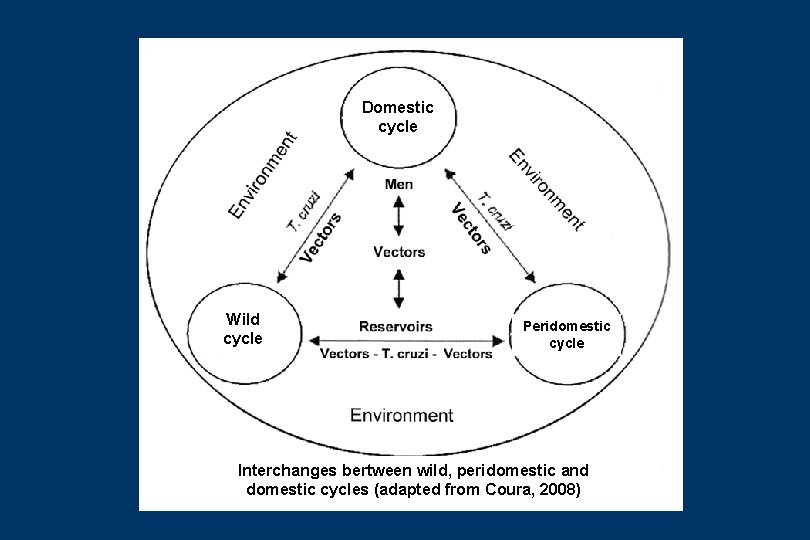
EPIDEMIOLOGY OF CHAGAS DISEASE (I) • The geographical distribuition of Chagas disease, its reservoirs and vectors, spread out from Southern US to Southern Argentina and Chile • It is currently estimaded that 90 millions of people in the Americas are exposed to infection and 15 millions are infected. These estimatives has been amusing and varies from 9 to 19 millions. • More than 130 species of potential vectors of T. cruzi are known, 5 of them are still importants in Brazil, after elimination of T. infestans: P. megistus, T. brasiliensis, T. pseudomaculata, T. sordida and R. brethesi • More than 100 species of wild reservoirs have been described among marsupials, xenarthra, bats, carnívores, logomorphs, rodents and primates. Among the domestic reservoirs, dogs, cats, domestic rats, mice and guinea-pigs are important to highlight • Thus we have three cycles interchanging chagasic infection: the wild cycle, the peridomestic cycle and the domestic cycle

Domestic cycle Wild cycle Peridomestic cycle Interchanges bertween wild, peridomestic and domestic cycles (adapted from Coura, 2008)

EPIDEMIOLOGY OF CHAGAS DISEASE (II) • The wild cycle of Chagas disease has exixted in nature for millions of years, but evidence of human infection has so far only been found in mummies from 4000 to 9000 years (Guhl et al 1999; Afderheide et al 2004) • The process of triatomines adaptation the human dwelling began with the agriculture cycle and was intensified with the deforestation for cattle raising • Transmission of Chagas infection evolved from enzootic of wild animals to an anthropozoonosis when man invaded wild ecotopes or when wild vectors or animals (Didelphis) invaded human dwellings and to zoonosis or anfixenosis after vectors adaptation to peridomestic and domestic cycles • The interchange between the wild, peridomestic and domestic cycles is a very complex two ways from each other

Palm trees habitat of triatomines and oppossum infected with Trypanosa cruzi invading huts in the Amazon region.

EPIDEMIOLOGY CHAGAS DISEASE (III) • The epidemiology of Chagas infection can be distrubuted in five groups of countries (Schmunis 1994, 2007; Dias & Coura 1997; Dias & Macedo 2005; Coura 2009) Group I – Which includes Argentina, Bolívia, Brazil, Chile, Ecuador, Honduras, Paraguay, Peru, Uruguay and Venezuela, presents domestic, peridomestic and wild cycles, with zones of high prevalence of human infection Group II – Which includes Colombia, Costa Rica and México, presents domestic, peridomestic and wild cycles with presence of human infection Group III – Which includes El Salvador, Guatemala, Nicaragua and Panamá, presents domestic, peridomestic and wild cycles, with little clinical information of human infection Group IV – Which includes Antilhas, Bahamas, Belize, Cuba, United States, Guyana, French Guyana, Haiti, Jamaica and Surinam, presents wild cycles with rare human autochthones cases Group V – Non-endemic countries as Australia, Canadá, European countries, Japan and other Asian countries and United States which receives migrants with Chagas disease from endemic countries

CONTROL AND SURVEILLANCE OF CHAGAS DISEASE (I) • Control of Chagas disease must be undertaken by interruption of the transmission though improving of dwellings, sanitary education, vector control, control of blood donnors, followed by permanent surveillance • The first steps is the domestic vector control by chemical insecticides and the blood donnors control by screening serology for T. cruzi infection, followed by improving dwellings and sanitary education of the exposed population • The chemical vector control is today basically using synthetic pyrethroids. Focal resistence of T. infestans in southern Bolivia and Salta, Argentina and R. prolixus in Venezuela has been detected • The serological control of blood donnors has been done in most countries. Areas without blood bank serological control, chemoprophylaxis using gentian violet or similar is indicated • Improving dwellings, sanitary education and surveillance has been neglected in most of countries

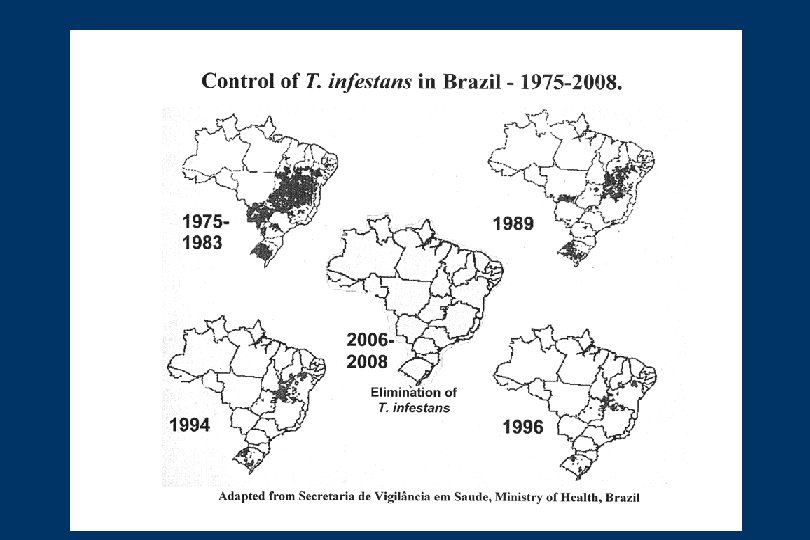
CONTROL AND SURVEILLANCE OF CHAGAS DISEASE (II) • Only in 1983 Chagas disease control was established in Brazil in a regular and continuous manner, and this extended to other countries thorugh the creation of the “Iniciatives” • The Southern Cone Initiative was created in 1991, Andean Countries in 1997, Central America and Mexico 1998 and Amazon countries in 2004 • Chile, Uruguay and Brazil eliminated T. infestans, the most important vector of T. cruzi infection. Paraguay is working on it and Argentina had some dificulty to control T. infestans • There is a possibility to eliminate R. prolixus from Central America. México must be stimulated to start Chagas disease control program. The other initiatives are improving • The initiatives created a new expectations for Latin America to control Chagas disease within the next 20 years. However, for these expectations to be confirmed there is a price: the controls programs must be constant and durable and eternal surveillance is requared

