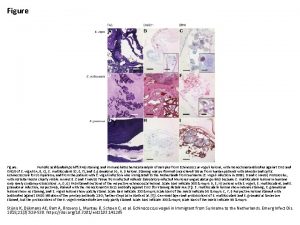
IMMUNOHISTOCHEMICAL DIFFERENTIAL DIAGNOSES IN UROLOGICAL PATHOLOGY An Overview



![P 504 S (Alpha-Methylacyl-Co. ARacemase [AMACR]) & Prostatic Tumors P 504 S+ Metastatic Prostatic P 504 S (Alpha-Methylacyl-Co. ARacemase [AMACR]) & Prostatic Tumors P 504 S+ Metastatic Prostatic](https://slidetodoc.com/presentation_image_h2/fd0196a07b01962776b77e3f159da77c/image-8.jpg)
![P 504 S (Alpha-Methylacyl-Co. ARacemase [AMACR]) & 34 BE 12 in Combination in Prostatic P 504 S (Alpha-Methylacyl-Co. ARacemase [AMACR]) & 34 BE 12 in Combination in Prostatic](https://slidetodoc.com/presentation_image_h2/fd0196a07b01962776b77e3f159da77c/image-12.jpg)








- Slides: 49

IMMUNOHISTOCHEMICAL DIFFERENTIAL DIAGNOSES IN UROLOGICAL PATHOLOGY: An Overview Mark R. Wick, M. D.

Diagnostic Urological Immunohistochemistry • The diagnosis of urological disease is a microcosm of surgical pathology at large, but with some selected differential diagnoses that are unique to the urogenital tract • As such, most immunohistochemical studies of urological lesions typically utilize antibody reagents that are common to diagnostic problems in other parts of the body • For those reasons, accurate & restricted morphological differential diagnosis is extremely important before application of immunostaining panels in urologic pathology

DIAGNOSTIC IMMUNOHISTOLOGY: CHOICE OF REAGENTS • Choices of panels of antibodies are dependent upon the specific differential diagnostic entities under consideration • Several antibodies appear in more than one panel, but their places in the relative sequences of interpretation (or the diagnoses that positivity yields) may differ from one setting to another • New antibodies may be substituted for old ones or used to supplement existing reagents in all panels

Useful Immunohistochemical Antibody Reagents in Non-Hematopoietic Urologic Pathology -Monospecific keratins 7, 18, 19, 20; Mo. Ab 34 BE 12 -Vimentin -Desmin -MS Actin -Collagen IV -PSAP -PSMA -ERP/PRP -ARP -GCDFP-15 -CA-125 -CEA -CDX 2 -TTF-1 -Thrombomodulin -EMA -p 63 -p 504 S -PLAP -S 100 -HMB 45 -MART-1 -AMACR -CD 10 -CD 26 -CD 30 -CD 56 -CD 99 -CD 117 -GATA 3 -Inhibin -PAX 8 -“RCC” -HEPPAR 1 -NB 84 -Synaptophysin -CGA -FLI-1

PROSTATIC NEOPLASMS

“Prostate-Specific” Markers • Prostatic specific antigen (PSA), Prostatic acid phosphatase (PSAP), and Prostate specific membrane antigen (PSMA) are the markers in current biochemical and immunohistochemical use, for identification of prostatic epithelial proliferations • PSA & PSAP antibodies are available from a variety of commercial sources; monoclonal antibodies should be used diagnostically to avoid unwanted cross-reactivity seen with heteroantisera • Prostate-specific membrane antigen (PSMA) is a 750 residue integral membrane glycoprotein recognized by a monoclonal antibody (7 E 11) from Hybritech Co.

How Specific Are “Prostate. Specific” Markers? • PSA and PSAP have been reported rarely in non-prostatic neoplasms, such as primary breast carcinoma, primary salivary duct carcinoma, and “pure” neuroendocrine carcinomas of various sites • PSMA is peculiarly expressed in the intratumoral endothelium of various neoplasms, but only by epithelial cells of the prostate and prostatic carcinomas
![P 504 S AlphaMethylacylCo ARacemase AMACR Prostatic Tumors P 504 S Metastatic Prostatic P 504 S (Alpha-Methylacyl-Co. ARacemase [AMACR]) & Prostatic Tumors P 504 S+ Metastatic Prostatic](https://slidetodoc.com/presentation_image_h2/fd0196a07b01962776b77e3f159da77c/image-8.jpg)
P 504 S (Alpha-Methylacyl-Co. ARacemase [AMACR]) & Prostatic Tumors P 504 S+ Metastatic Prostatic Carcinoma in Lymph Node • Cytoplasmic protein identified by c. DNA library subtraction with microarray screening, from prostatic carcinoma • AMACR functions in the betaoxidation of branched-chain fatty acids • Anti-P 504 S is marketed by Zeta Co. , Sierra Madre, CA, USA (murine monoclonal antibody) • Complete organ/tissue distribution not yet studied, but urothelium & urothelial tumors are P 504 S-negative

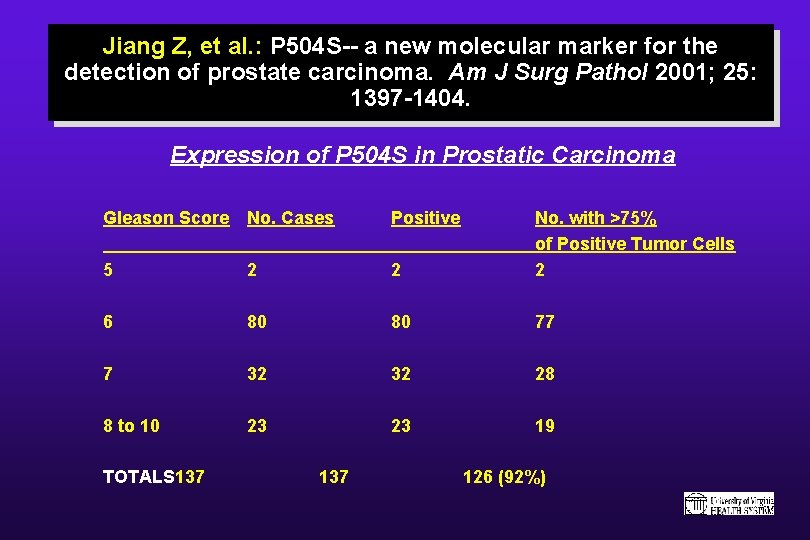
Jiang Z, et al. : P 504 S-- a new molecular marker for the detection of prostate carcinoma. Am J Surg Pathol 2001; 25: 1397 -1404. Expression of P 504 S in Prostatic Carcinoma Gleason Score No. Cases Positive 5 2 2 No. with >75% of Positive Tumor Cells 2 6 80 80 77 7 32 32 28 8 to 10 23 23 19 TOTALS 137 126 (92%)

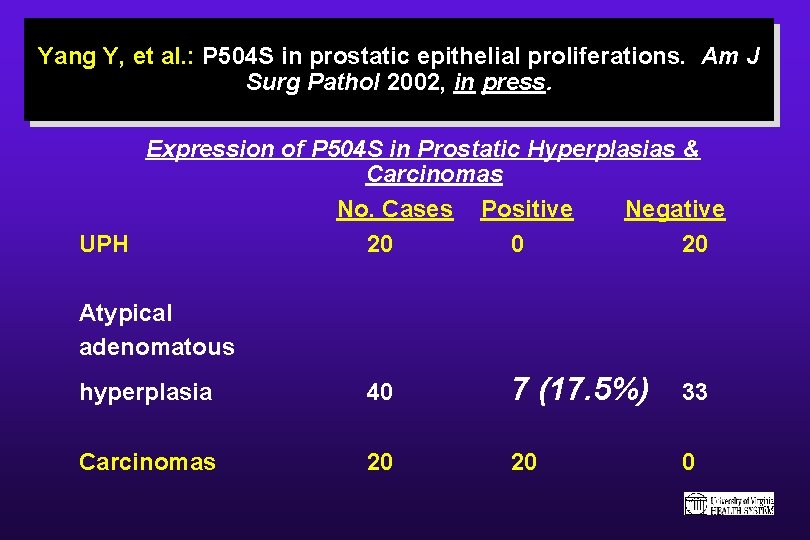
Yang Y, et al. : P 504 S in prostatic epithelial proliferations. Am J Surg Pathol 2002, in press. Expression of P 504 S in Prostatic Hyperplasias & Carcinomas No. Cases Positive Negative UPH 20 0 20 Atypical adenomatous hyperplasia 40 7 (17. 5%) 33 Carcinomas 20 20 0

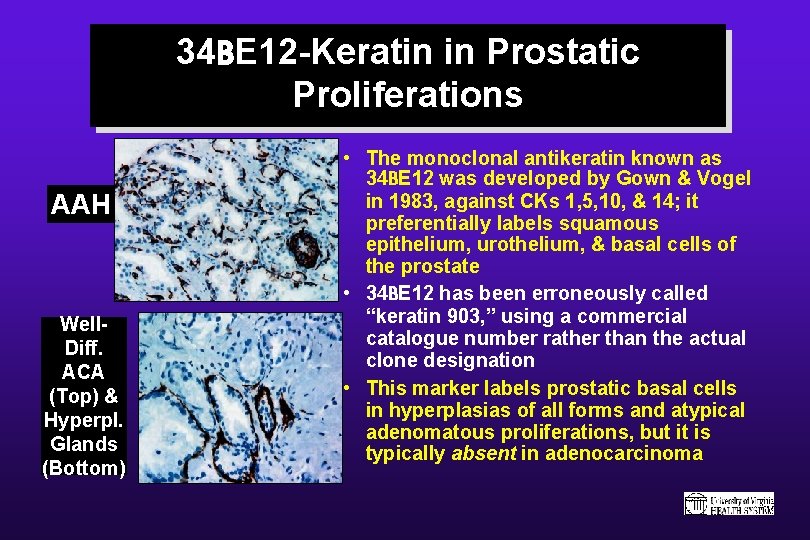
34 BE 12 -Keratin in Prostatic Proliferations AAH Well. Diff. ACA (Top) & Hyperpl. Glands (Bottom) • The monoclonal antikeratin known as 34 BE 12 was developed by Gown & Vogel in 1983, against CKs 1, 5, 10, & 14; it preferentially labels squamous epithelium, urothelium, & basal cells of the prostate • 34 BE 12 has been erroneously called “keratin 903, ” using a commercial catalogue number rather than the actual clone designation • This marker labels prostatic basal cells in hyperplasias of all forms and atypical adenomatous proliferations, but it is typically absent in adenocarcinoma
![P 504 S AlphaMethylacylCo ARacemase AMACR 34 BE 12 in Combination in Prostatic P 504 S (Alpha-Methylacyl-Co. ARacemase [AMACR]) & 34 BE 12 in Combination in Prostatic](https://slidetodoc.com/presentation_image_h2/fd0196a07b01962776b77e3f159da77c/image-12.jpg)
P 504 S (Alpha-Methylacyl-Co. ARacemase [AMACR]) & 34 BE 12 in Combination in Prostatic Biopsies 34 BE 12 P 504 S • P 504 S can be used complementarily with 34 BE 12 -keratin to help to distinguish welldifferentiated prostatic adenocarcinoma from atypical benign glandular proliferations • Those 2 markers produce “mirror image” results with respect to one another

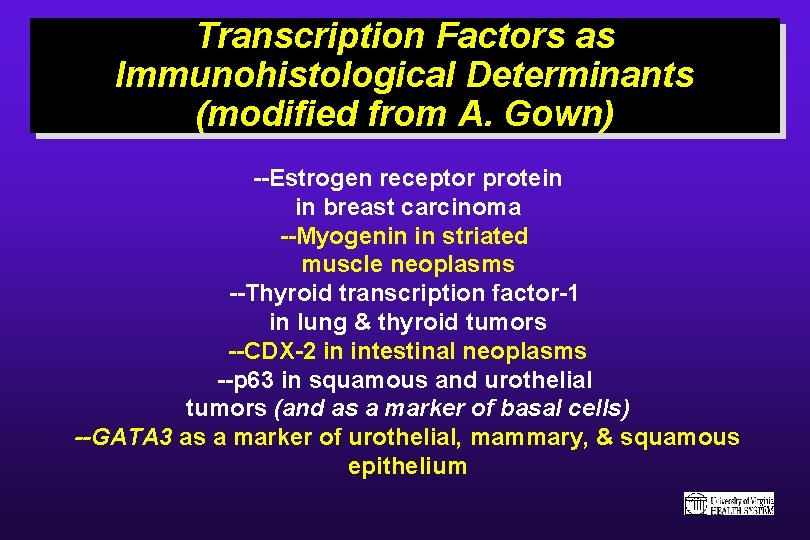
Transcription Factors as Immunohistological Determinants (modified from A. Gown) --Estrogen receptor protein in breast carcinoma --Myogenin in striated muscle neoplasms --Thyroid transcription factor-1 in lung & thyroid tumors --CDX-2 in intestinal neoplasms --p 63 in squamous and urothelial tumors (and as a marker of basal cells) --GATA 3 as a marker of urothelial, mammary, & squamous epithelium

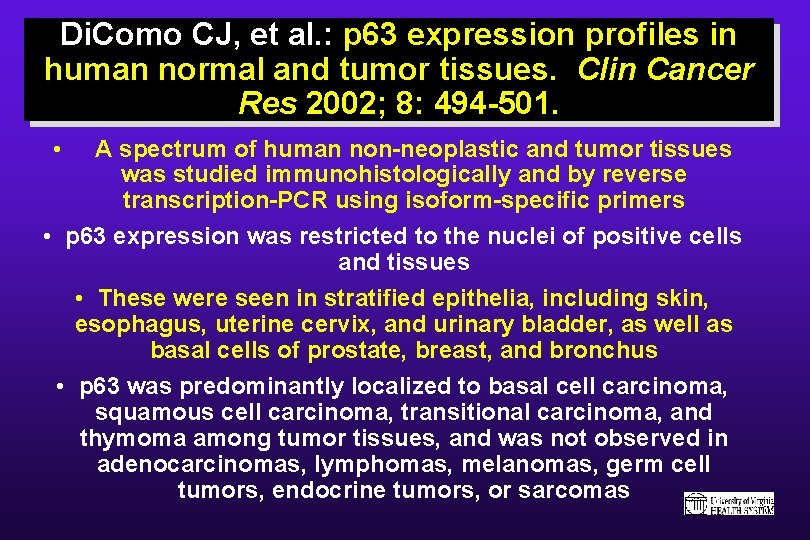
Di. Como CJ, et al. : p 63 expression profiles in human normal and tumor tissues. Clin Cancer Res 2002; 8: 494 -501. • A spectrum of human non-neoplastic and tumor tissues was studied immunohistologically and by reverse transcription-PCR using isoform-specific primers • p 63 expression was restricted to the nuclei of positive cells and tissues • These were seen in stratified epithelia, including skin, esophagus, uterine cervix, and urinary bladder, as well as basal cells of prostate, breast, and bronchus • p 63 was predominantly localized to basal cell carcinoma, squamous cell carcinoma, transitional carcinoma, and thymoma among tumor tissues, and was not observed in adenocarcinomas, lymphomas, melanomas, germ cell tumors, endocrine tumors, or sarcomas

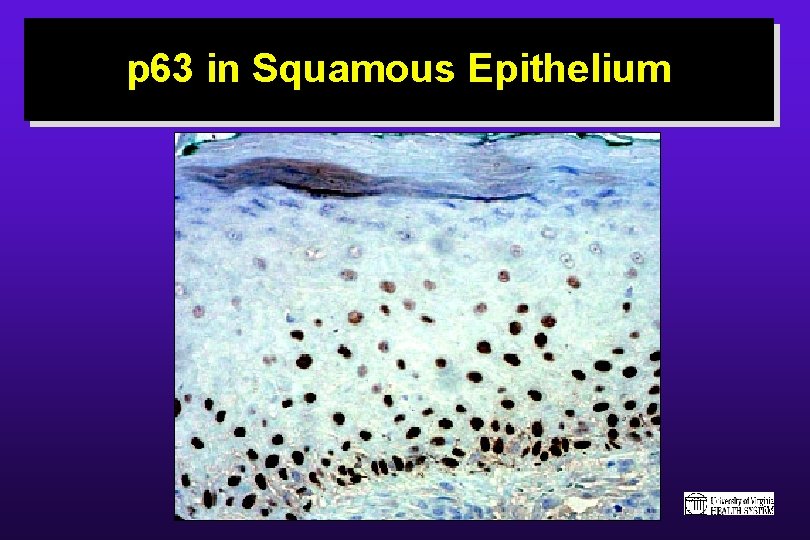
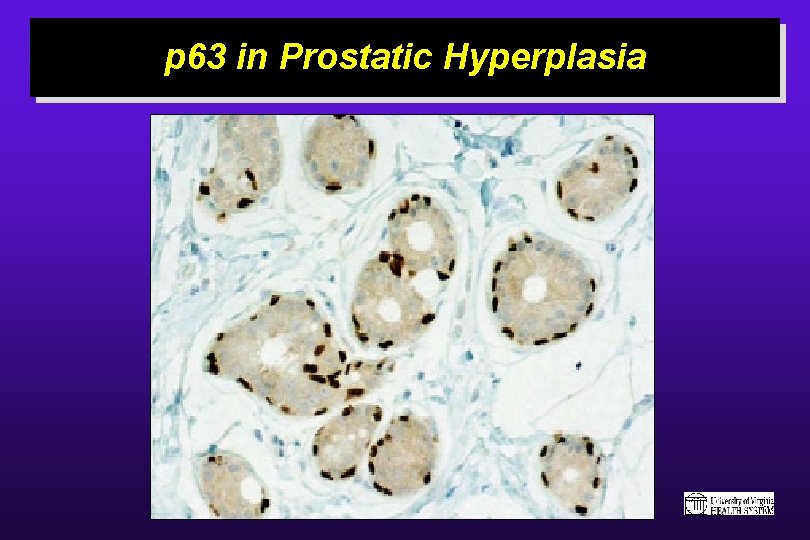
p 63 in Squamous Epithelium

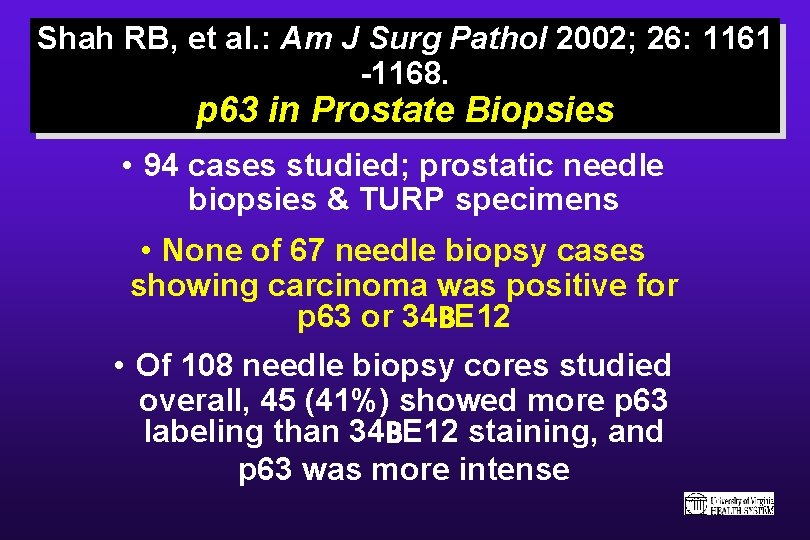
p 63: Another Marker of Prostatic Basal Cells Comparison of the Basal Cell-Specific Markers, 34 beta. E 12 and p 63, in the Diagnosis of Prostate Cancer. Shah RB, Zhou M, Le. Blanc M, Snyder M, Rubin MA. Departments of Pathology and Urology, University of Michigan School of Medicine and Comprenhensive Cancer Center, Ann Arbor, Michigan 48109, USA Am J Surg Pathol 2002; 26: 1161 -1168.

Shah RB, et al. : Am J Surg Pathol 2002; 26: 1161 -1168. p 63 in Prostate Biopsies • 94 cases studied; prostatic needle biopsies & TURP specimens • None of 67 needle biopsy cases showing carcinoma was positive for p 63 or 34 BE 12 • Of 108 needle biopsy cores studied overall, 45 (41%) showed more p 63 labeling than 34 BE 12 staining, and p 63 was more intense

p 63 in Prostatic Hyperplasia

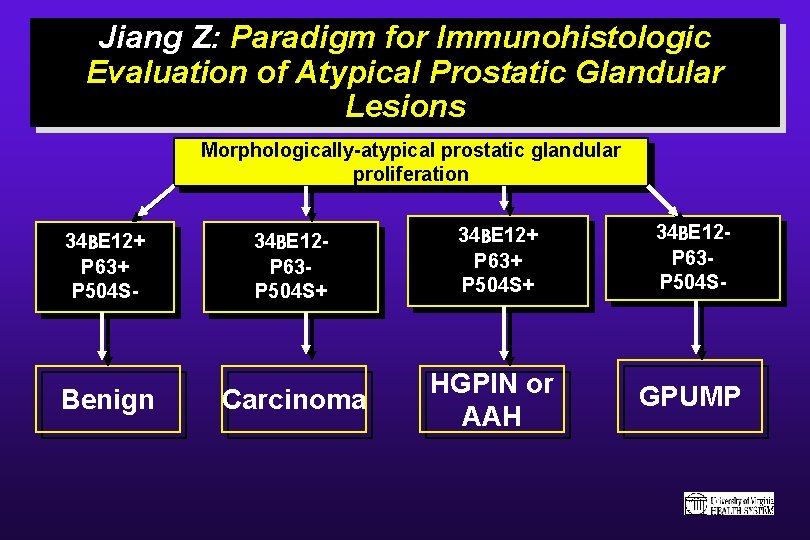
Jiang Z: Paradigm for Immunohistologic Evaluation of Atypical Prostatic Glandular Lesions Morphologically-atypical prostatic glandular proliferation 34 BE 12+ P 63+ P 504 S- Benign 34 BE 12 P 63 P 504 S+ Carcinoma 34 BE 12+ P 63+ P 504 S+ HGPIN or AAH 34 BE 12 P 63 P 504 S- GPUMP

Practical Differential Diagnoses Involving Prostatic Carcinoma & Immunohistochemistry • Prostatic carcinoma vs. poorly-differentiated TCC • Prostatic acinar carcinoma vs. “pure” neuroendocrine CA • Prostatic carcinoma vs. poorly-differentiated colonic CA • Prostatic carcinoma vs. Cowper’s glands or seminal vesicle • Prostatic carcinoma vs. nephrogenic metaplasia (adenoma) • Prostatic acinar carcinoma vs. clear-cell adenocarcinoma • Metastatic prostatic carcinoma vs. 1 o or 2 o salivary CA • Metastatic prostatic carcinoma vs. 1 o or 2 o breast CA

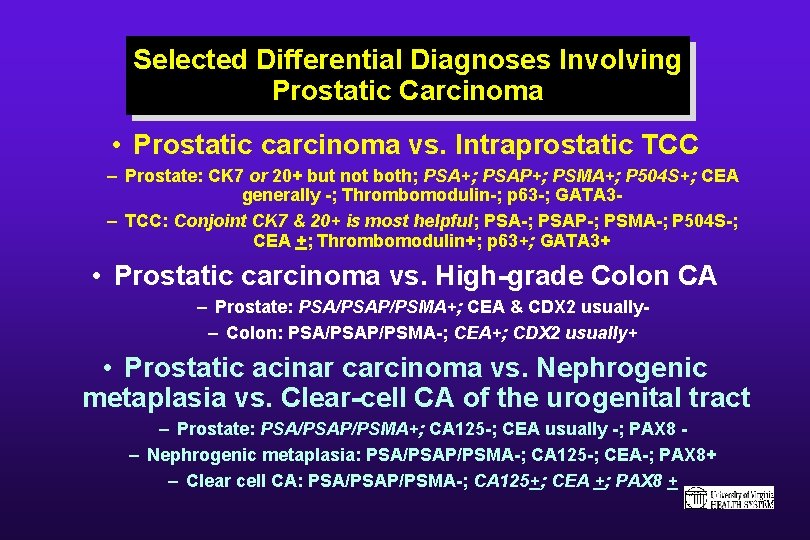
Selected Differential Diagnoses Involving Prostatic Carcinoma • Prostatic carcinoma vs. Intraprostatic TCC – Prostate: CK 7 or 20+ but not both; PSA+; PSAP+; PSMA+; P 504 S+; CEA generally -; Thrombomodulin-; p 63 -; GATA 3– TCC: Conjoint CK 7 & 20+ is most helpful; PSA-; PSAP-; PSMA-; P 504 S-; CEA +; Thrombomodulin+; p 63+; GATA 3+ • Prostatic carcinoma vs. High-grade Colon CA – Prostate: PSA/PSAP/PSMA+; CEA & CDX 2 usually– Colon: PSA/PSAP/PSMA-; CEA+; CDX 2 usually+ • Prostatic acinar carcinoma vs. Nephrogenic metaplasia vs. Clear-cell CA of the urogenital tract – Prostate: PSA/PSAP/PSMA+; CA 125 -; CEA usually -; PAX 8 – Nephrogenic metaplasia: PSA/PSAP/PSMA-; CA 125 -; CEA-; PAX 8+ – Clear cell CA: PSA/PSAP/PSMA-; CA 125+; CEA +; PAX 8 +

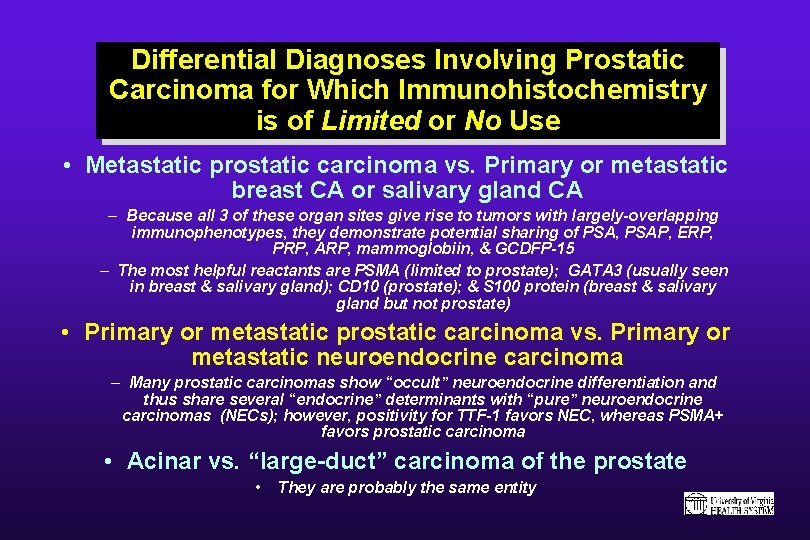
Differential Diagnoses Involving Prostatic Carcinoma for Which Immunohistochemistry is of Limited or No Use • Metastatic prostatic carcinoma vs. Primary or metastatic breast CA or salivary gland CA – Because all 3 of these organ sites give rise to tumors with largely-overlapping immunophenotypes, they demonstrate potential sharing of PSA, PSAP, ERP, PRP, ARP, mammoglobiin, & GCDFP-15 – The most helpful reactants are PSMA (limited to prostate); GATA 3 (usually seen in breast & salivary gland); CD 10 (prostate); & S 100 protein (breast & salivary gland but not prostate) • Primary or metastatic prostatic carcinoma vs. Primary or metastatic neuroendocrine carcinoma – Many prostatic carcinomas show “occult” neuroendocrine differentiation and thus share several “endocrine” determinants with “pure” neuroendocrine carcinomas (NECs); however, positivity for TTF-1 favors NEC, whereas PSMA+ favors prostatic carcinoma • Acinar vs. “large-duct” carcinoma of the prostate • They are probably the same entity

ADULT RENAL NEOPLASMS

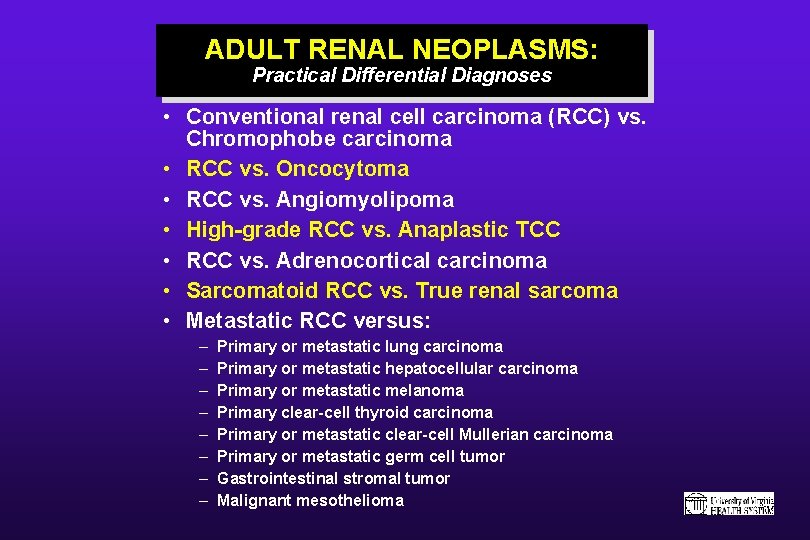
ADULT RENAL NEOPLASMS: Practical Differential Diagnoses • Conventional renal cell carcinoma (RCC) vs. Chromophobe carcinoma • RCC vs. Oncocytoma • RCC vs. Angiomyolipoma • High-grade RCC vs. Anaplastic TCC • RCC vs. Adrenocortical carcinoma • Sarcomatoid RCC vs. True renal sarcoma • Metastatic RCC versus: – – – – Primary or metastatic lung carcinoma Primary or metastatic hepatocellular carcinoma Primary or metastatic melanoma Primary clear-cell thyroid carcinoma Primary or metastatic clear-cell Mullerian carcinoma Primary or metastatic germ cell tumor Gastrointestinal stromal tumor Malignant mesothelioma

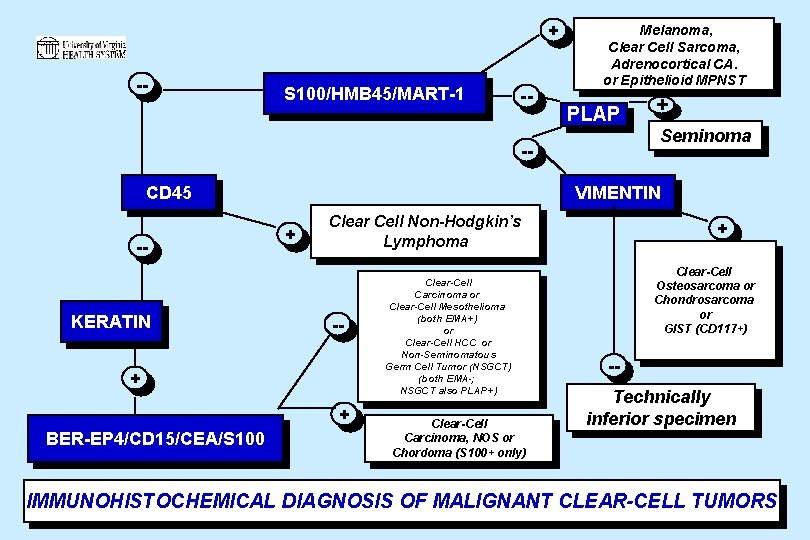
+ -- S 100/HMB 45/MART-1 -- Melanoma, Clear Cell Sarcoma, Adrenocortical CA. or Epithelioid MPNST PLAP -CD 45 -- KERATIN Seminoma VIMENTIN + Clear Cell Non-Hodgkin’s Lymphoma -- + + BER-EP 4/CD 15/CEA/S 100 + Clear-Cell Carcinoma or Clear-Cell Mesothelioma (both EMA+) or Clear-Cell HCC or Non-Seminomatous Germ Cell Tumor (NSGCT) (both EMA-; NSGCT also PLAP+) Clear-Cell Carcinoma, NOS or Chordoma (S 100+ only) + Clear-Cell Osteosarcoma or Chondrosarcoma or GIST (CD 117+) -Technically inferior specimen IMMUNOHISTOCHEMICAL DIAGNOSIS OF MALIGNANT CLEAR-CELL TUMORS

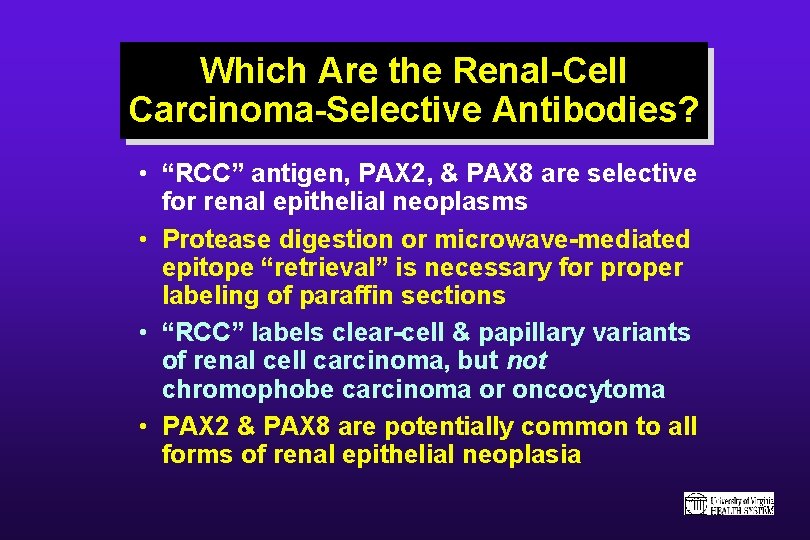
Which Are the Renal-Cell Carcinoma-Selective Antibodies? • “RCC” antigen, PAX 2, & PAX 8 are selective for renal epithelial neoplasms • Protease digestion or microwave-mediated epitope “retrieval” is necessary for proper labeling of paraffin sections • “RCC” labels clear-cell & papillary variants of renal cell carcinoma, but not chromophobe carcinoma or oncocytoma • PAX 2 & PAX 8 are potentially common to all forms of renal epithelial neoplasia







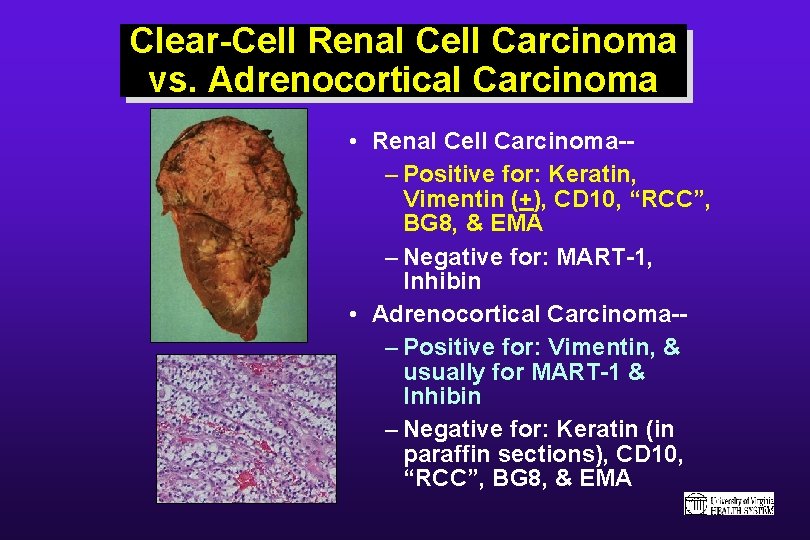
Clear-Cell Renal Cell Carcinoma vs. Adrenocortical Carcinoma • Renal Cell Carcinoma-– Positive for: Keratin, Vimentin (+), CD 10, “RCC”, BG 8, & EMA – Negative for: MART-1, Inhibin • Adrenocortical Carcinoma-– Positive for: Vimentin, & usually for MART-1 & Inhibin – Negative for: Keratin (in paraffin sections), CD 10, “RCC”, BG 8, & EMA

Sarcomatoid Renal Cell Carcinoma vs. True Renal Sarcoma • Sarcomatoid carcinoma is a “final common pathway” of clonal evolution, with or without divergent differentiation. Thus, sarcomatoid RCCs (SRCCs) show a marked diminution of reactivity for keratin and EMA, augmentation of vimentin labeling, and the possible appearance of “heterologous” markers such as desmin, muscle-specific actin, & osteonectin • True intraparenchymal renal sarcomas are extraordinarily rare, and represent diagnoses of ultimate exclusion • Immunohistochemical diagnosis on small tumor biopsies is especially treacherous

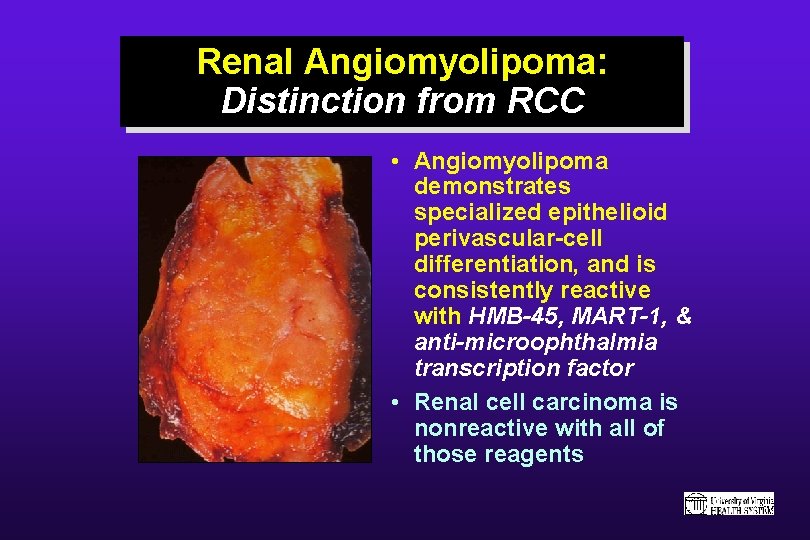
Renal Angiomyolipoma: Distinction from RCC • Angiomyolipoma demonstrates specialized epithelioid perivascular-cell differentiation, and is consistently reactive with HMB-45, MART-1, & anti-microophthalmia transcription factor • Renal cell carcinoma is nonreactive with all of those reagents

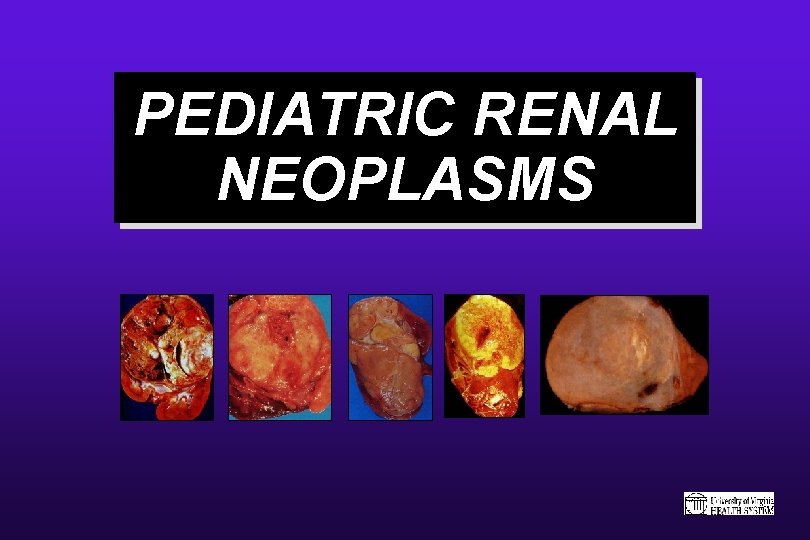
PEDIATRIC RENAL NEOPLASMS

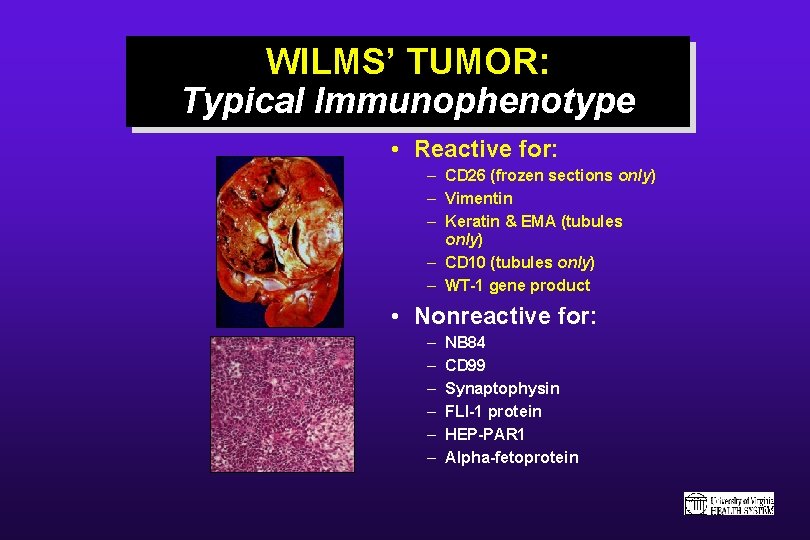
WILMS’ TUMOR: Typical Immunophenotype • Reactive for: – CD 26 (frozen sections only) – Vimentin – Keratin & EMA (tubules only) – CD 10 (tubules only) – WT-1 gene product • Nonreactive for: – – – NB 84 CD 99 Synaptophysin FLI-1 protein HEP-PAR 1 Alpha-fetoprotein

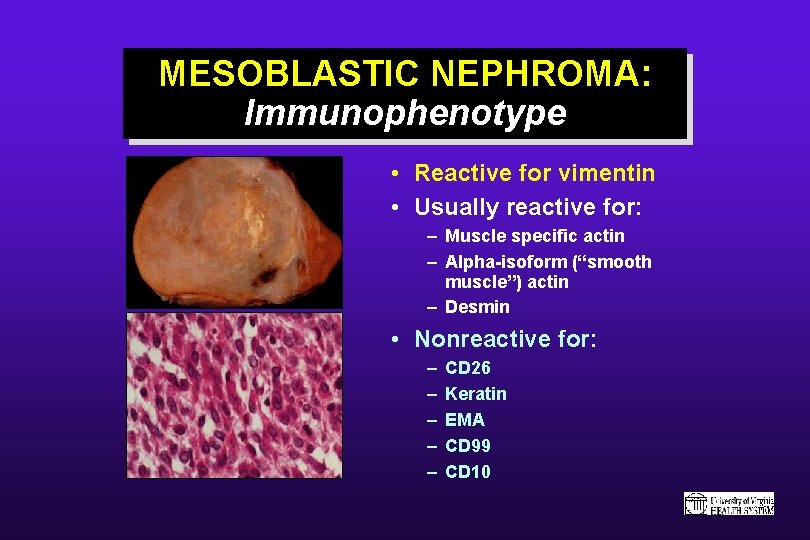
MESOBLASTIC NEPHROMA: Immunophenotype • Reactive for vimentin • Usually reactive for: – Muscle specific actin – Alpha-isoform (“smooth muscle”) actin – Desmin • Nonreactive for: – – – CD 26 Keratin EMA CD 99 CD 10

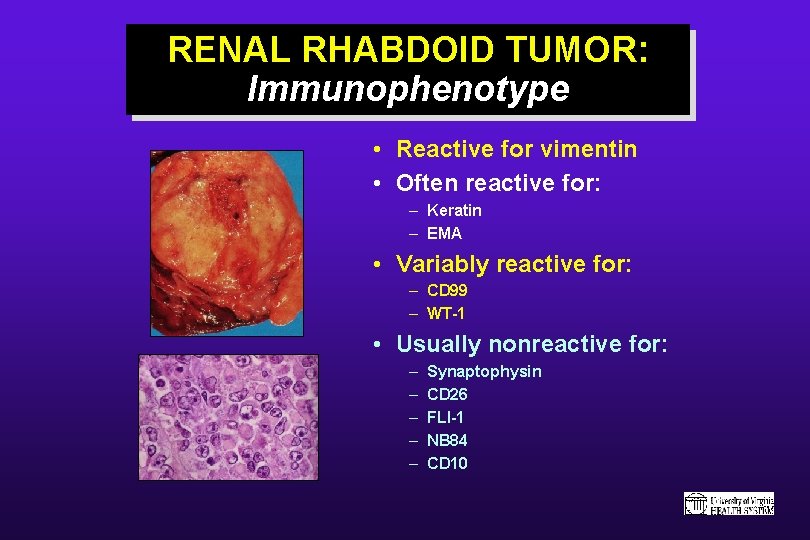
RENAL RHABDOID TUMOR: Immunophenotype • Reactive for vimentin • Often reactive for: – Keratin – EMA • Variably reactive for: – CD 99 – WT-1 • Usually nonreactive for: – – – Synaptophysin CD 26 FLI-1 NB 84 CD 10

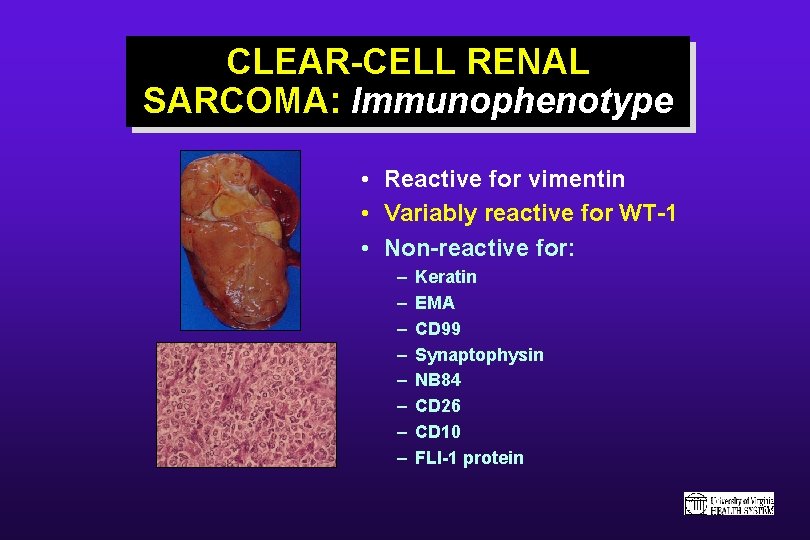
CLEAR-CELL RENAL SARCOMA: Immunophenotype • Reactive for vimentin • Variably reactive for WT-1 • Non-reactive for: – – – – Keratin EMA CD 99 Synaptophysin NB 84 CD 26 CD 10 FLI-1 protein

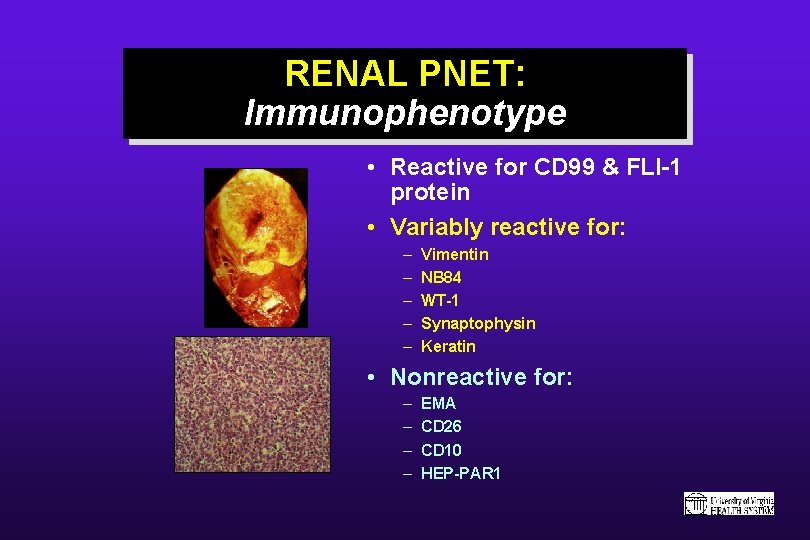
RENAL PNET: Immunophenotype • Reactive for CD 99 & FLI-1 protein • Variably reactive for: – – – Vimentin NB 84 WT-1 Synaptophysin Keratin • Nonreactive for: – – EMA CD 26 CD 10 HEP-PAR 1

TRANSITIONAL CELL CARCINOMA VARIANTS

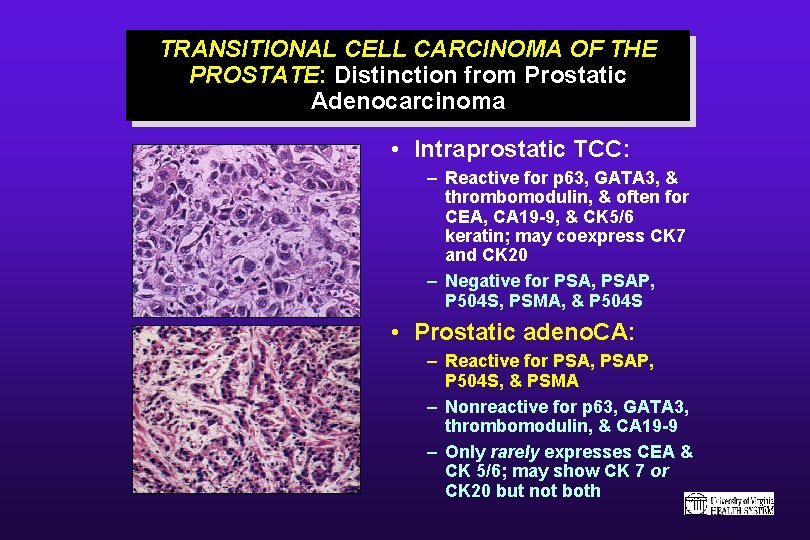
TRANSITIONAL CELL CARCINOMA OF THE PROSTATE: Distinction from Prostatic Adenocarcinoma • Intraprostatic TCC: – Reactive for p 63, GATA 3, & thrombomodulin, & often for CEA, CA 19 -9, & CK 5/6 keratin; may coexpress CK 7 and CK 20 – Negative for PSA, PSAP, P 504 S, PSMA, & P 504 S • Prostatic adeno. CA: – Reactive for PSA, PSAP, P 504 S, & PSMA – Nonreactive for p 63, GATA 3, thrombomodulin, & CA 19 -9 – Only rarely expresses CEA & CK 5/6; may show CK 7 or CK 20 but not both

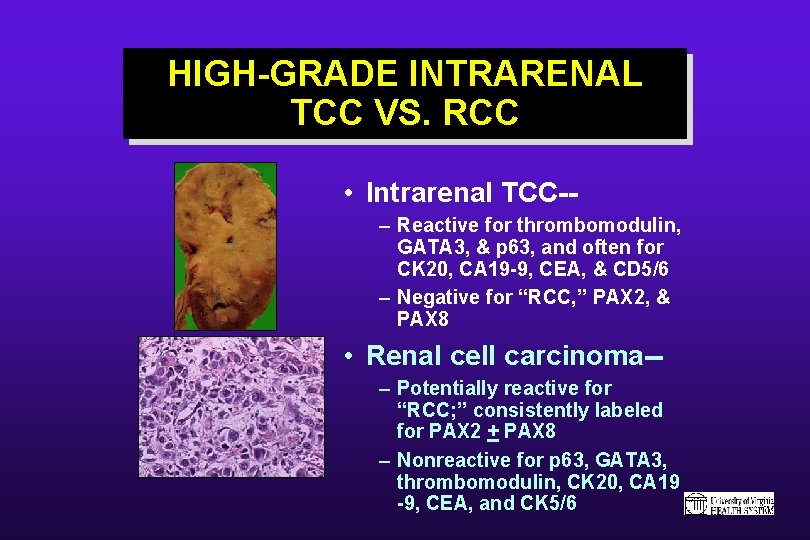
HIGH-GRADE INTRARENAL TCC VS. RCC • Intrarenal TCC-– Reactive for thrombomodulin, GATA 3, & p 63, and often for CK 20, CA 19 -9, CEA, & CD 5/6 – Negative for “RCC, ” PAX 2, & PAX 8 • Renal cell carcinoma-– Potentially reactive for “RCC; ” consistently labeled for PAX 2 + PAX 8 – Nonreactive for p 63, GATA 3, thrombomodulin, CK 20, CA 19 -9, CEA, and CK 5/6

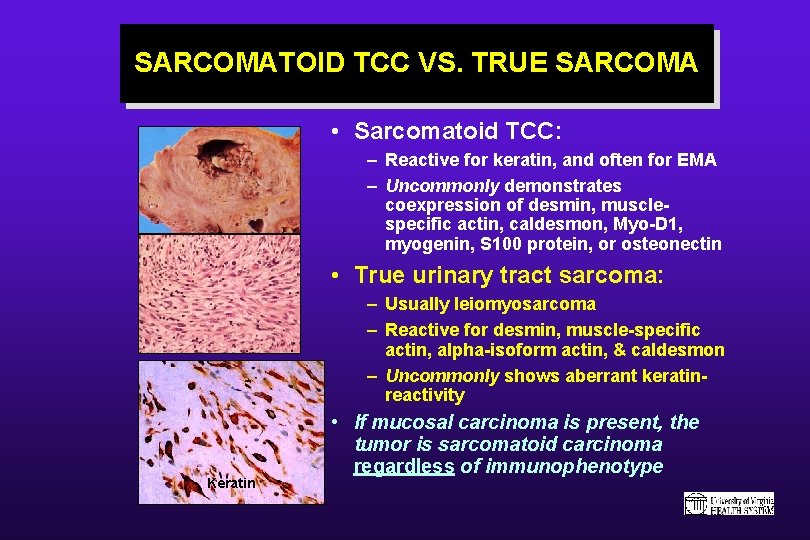
SARCOMATOID TCC VS. TRUE SARCOMA • Sarcomatoid TCC: – Reactive for keratin, and often for EMA – Uncommonly demonstrates coexpression of desmin, musclespecific actin, caldesmon, Myo-D 1, myogenin, S 100 protein, or osteonectin • True urinary tract sarcoma: – Usually leiomyosarcoma – Reactive for desmin, muscle-specific actin, alpha-isoform actin, & caldesmon – Uncommonly shows aberrant keratinreactivity Keratin • If mucosal carcinoma is present, the tumor is sarcomatoid carcinoma regardless of immunophenotype

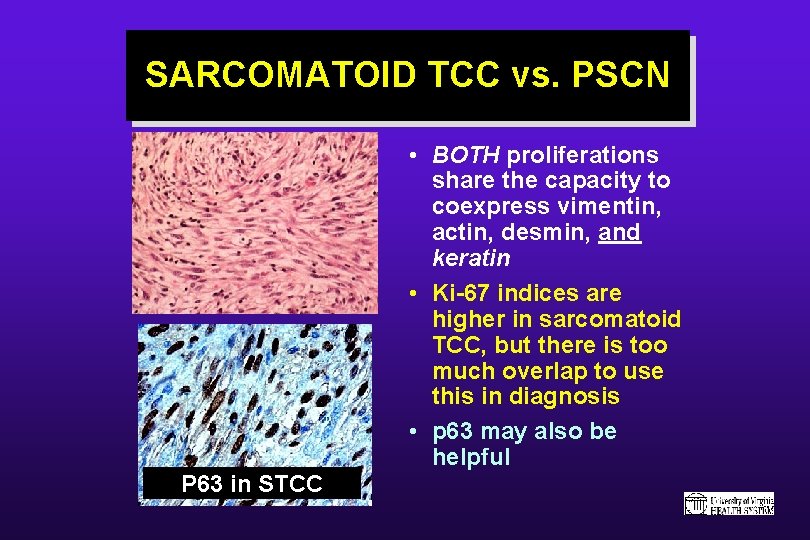
SARCOMATOID TCC vs. PSCN • BOTH proliferations share the capacity to coexpress vimentin, actin, desmin, and keratin • Ki-67 indices are higher in sarcomatoid TCC, but there is too much overlap to use this in diagnosis • p 63 may also be helpful P 63 in STCC

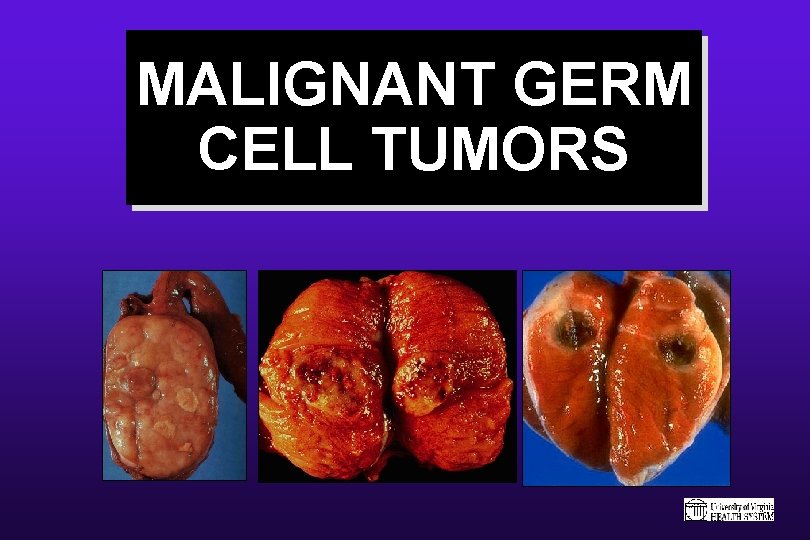
MALIGNANT GERM CELL TUMORS

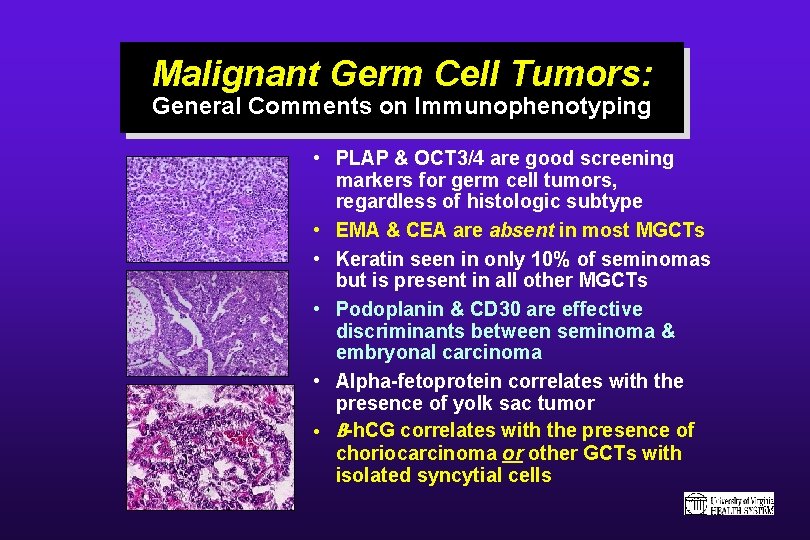
Malignant Germ Cell Tumors: General Comments on Immunophenotyping • PLAP & OCT 3/4 are good screening markers for germ cell tumors, regardless of histologic subtype • EMA & CEA are absent in most MGCTs • Keratin seen in only 10% of seminomas but is present in all other MGCTs • Podoplanin & CD 30 are effective discriminants between seminoma & embryonal carcinoma • Alpha-fetoprotein correlates with the presence of yolk sac tumor • B-h. CG correlates with the presence of choriocarcinoma or other GCTs with isolated syncytial cells

 Nursing process objectives
Nursing process objectives Female reproductive system pathology
Female reproductive system pathology Plant pathology
Plant pathology In pathology
In pathology E3bp
E3bp Pathway pansitopenia
Pathway pansitopenia Plant pathology
Plant pathology Xanthelasma pathology
Xanthelasma pathology Oral pathology
Oral pathology Corneal nebulae
Corneal nebulae Kidney pathology
Kidney pathology Pancreatitis pathophysiology
Pancreatitis pathophysiology Plant pathology
Plant pathology Emricon
Emricon Quality management in anatomic pathology
Quality management in anatomic pathology Name the five lesions associated with hiv/aids chapter 17
Name the five lesions associated with hiv/aids chapter 17 Banff pathology course
Banff pathology course Nanometer to millimeter
Nanometer to millimeter Types of pathology
Types of pathology Branches of pathology
Branches of pathology Gross pathology definition
Gross pathology definition Glomerulonephritis
Glomerulonephritis Utah web path
Utah web path Pathology conference
Pathology conference Spontaneous miscarriage
Spontaneous miscarriage Bcc pathology
Bcc pathology Budd
Budd Clinical pathology definition
Clinical pathology definition Forensic pathology quiz
Forensic pathology quiz Introduction to cryptology
Introduction to cryptology Thyroid pathology
Thyroid pathology Mucoepidermoid carcinoma pathology outlines
Mucoepidermoid carcinoma pathology outlines Rph pathology
Rph pathology Heart
Heart Female reproductive system pathology
Female reproductive system pathology Nabl 161
Nabl 161 Plant pathology
Plant pathology Leeds pathology tests and tubes
Leeds pathology tests and tubes Raphe palatini
Raphe palatini Social pathology
Social pathology Thyroid follicle histology
Thyroid follicle histology Epath pathology
Epath pathology Pronator teres
Pronator teres History of fungicides
History of fungicides Nutmeg liver slideshare
Nutmeg liver slideshare Pathology
Pathology Plant pathology
Plant pathology Pathology of blood and urine ppt
Pathology of blood and urine ppt Tennis racket appearance in oral pathology
Tennis racket appearance in oral pathology Oral pathology
Oral pathology