Immune Responses to Viral Infections Interferons Proteins synthesized


![Inhibitory receptors contain ITIM (immunoreceptor tyrosine-based inhibitory motif) in their cytoplasmic domains. [I/V]XYXXL: Y Inhibitory receptors contain ITIM (immunoreceptor tyrosine-based inhibitory motif) in their cytoplasmic domains. [I/V]XYXXL: Y](https://slidetodoc.com/presentation_image_h2/4627a2cb187d98d2a5fbe64850f0660d/image-11.jpg)




- Slides: 33

Immune Responses to Viral Infections


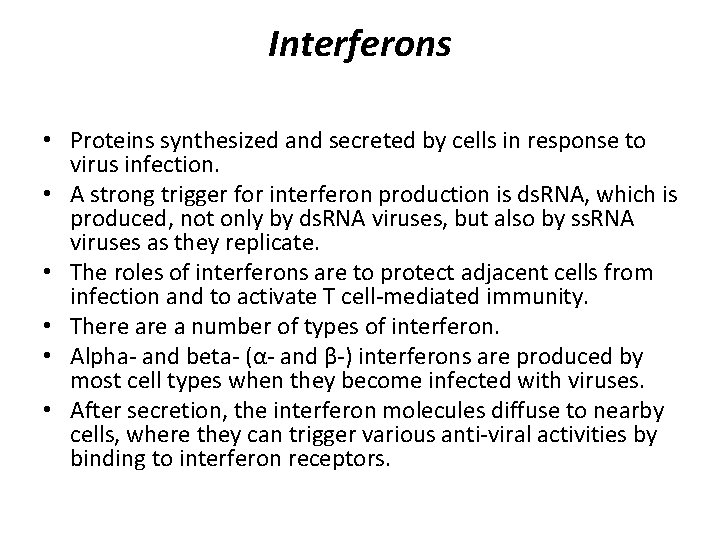
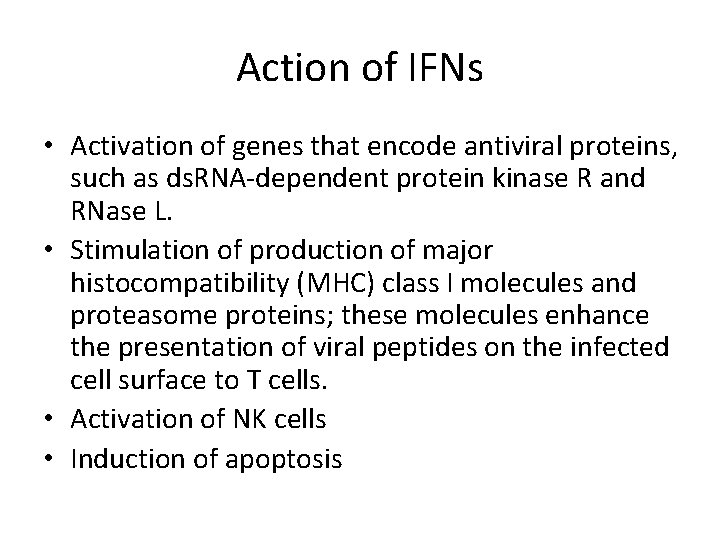
Interferons • Proteins synthesized and secreted by cells in response to virus infection. • A strong trigger for interferon production is ds. RNA, which is produced, not only by ds. RNA viruses, but also by ss. RNA viruses as they replicate. • The roles of interferons are to protect adjacent cells from infection and to activate T cell-mediated immunity. • There a number of types of interferon. • Alpha- and beta- (α- and β-) interferons are produced by most cell types when they become infected with viruses. • After secretion, the interferon molecules diffuse to nearby cells, where they can trigger various anti-viral activities by binding to interferon receptors.

Action of IFNs • Activation of genes that encode antiviral proteins, such as ds. RNA-dependent protein kinase R and RNase L. • Stimulation of production of major histocompatibility (MHC) class I molecules and proteasome proteins; these molecules enhance the presentation of viral peptides on the infected cell surface to T cells. • Activation of NK cells • Induction of apoptosis

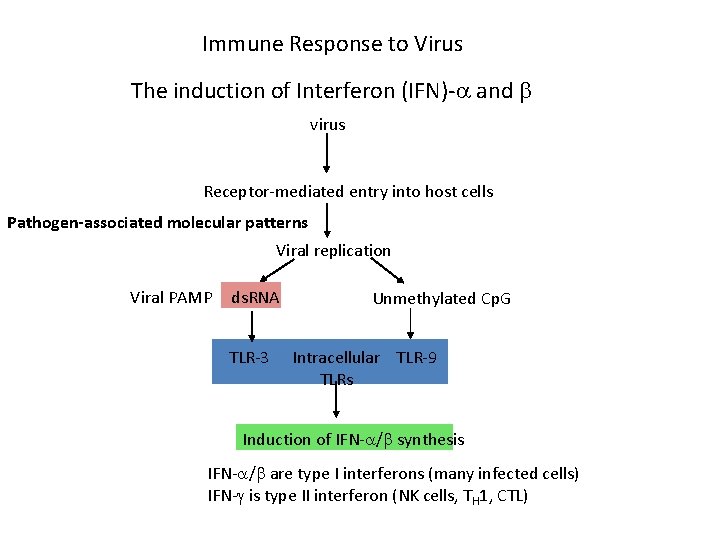
Immune Response to Virus The induction of Interferon (IFN)- and virus Receptor-mediated entry into host cells Pathogen-associated molecular patterns Viral replication Viral PAMP ds. RNA TLR-3 Unmethylated Cp. G Intracellular TLR-9 TLRs Induction of IFN- / synthesis IFN- / are type I interferons (many infected cells) IFN- is type II interferon (NK cells, TH 1, CTL)

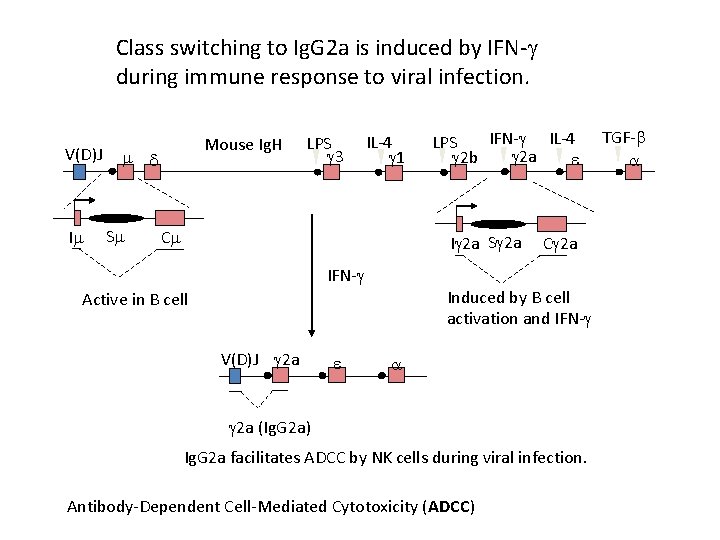
Class switching to Ig. G 2 a is induced by IFN- during immune response to viral infection. V(D)J I Mouse Ig. H S LPS 3 IL-4 1 C IFN- IL-4 LPS 2 a 2 b I 2 a S 2 a IFN- Induced by B cell activation and IFN- Active in B cell V(D)J 2 a C 2 a 2 a (Ig. G 2 a) Ig. G 2 a facilitates ADCC by NK cells during viral infection. Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC) TGF-

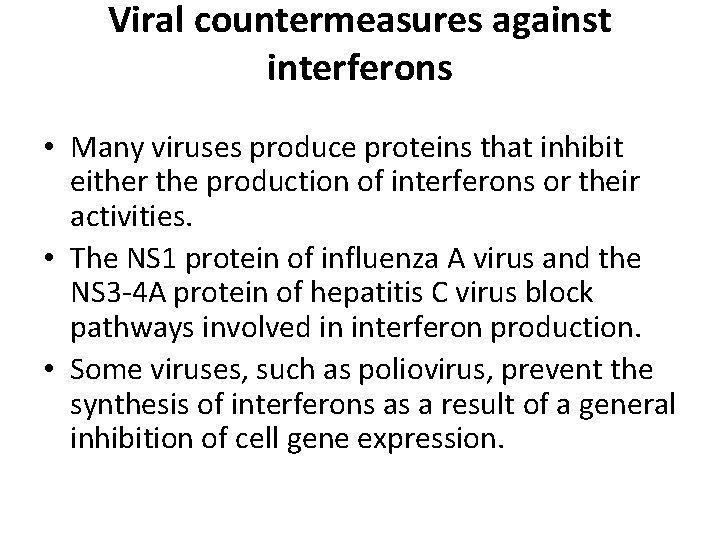
Viral countermeasures against interferons • Many viruses produce proteins that inhibit either the production of interferons or their activities. • The NS 1 protein of influenza A virus and the NS 3 -4 A protein of hepatitis C virus block pathways involved in interferon production. • Some viruses, such as poliovirus, prevent the synthesis of interferons as a result of a general inhibition of cell gene expression.

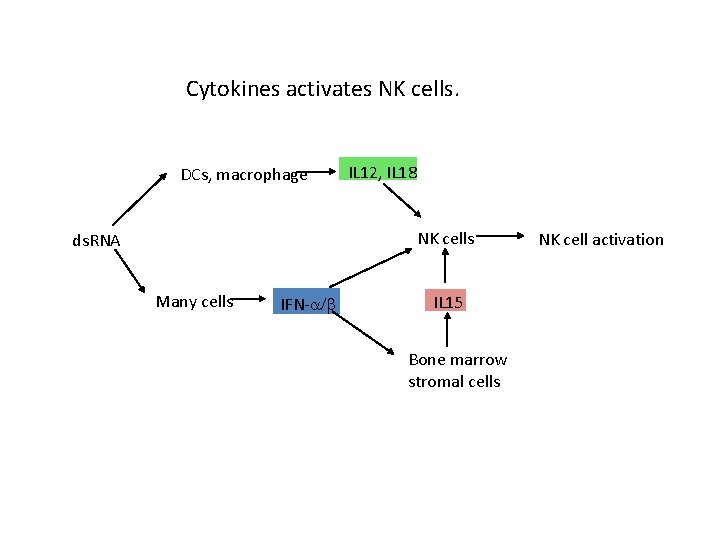
Cytokines activates NK cells. DCs, macrophage IL 12, IL 18 NK cells ds. RNA Many cells IFN- IL 15 Bone marrow stromal cells NK cell activation

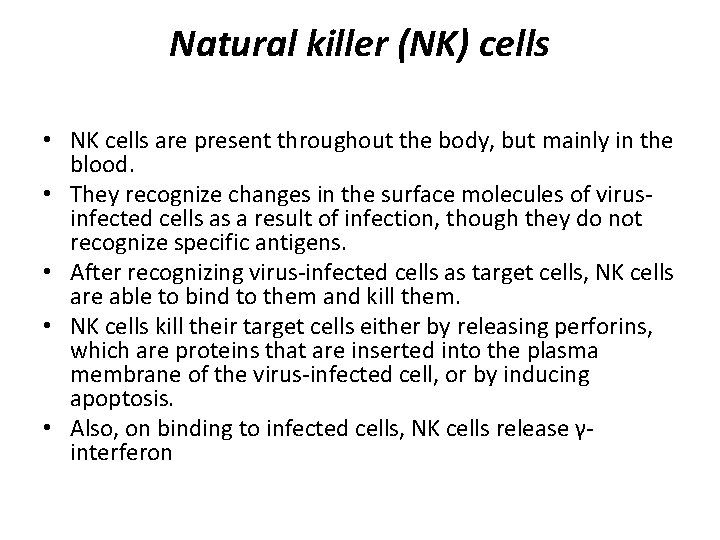
Natural killer (NK) cells • NK cells are present throughout the body, but mainly in the blood. • They recognize changes in the surface molecules of virusinfected cells as a result of infection, though they do not recognize specific antigens. • After recognizing virus-infected cells as target cells, NK cells are able to bind to them and kill them. • NK cells kill their target cells either by releasing perforins, which are proteins that are inserted into the plasma membrane of the virus-infected cell, or by inducing apoptosis. • Also, on binding to infected cells, NK cells release γinterferon

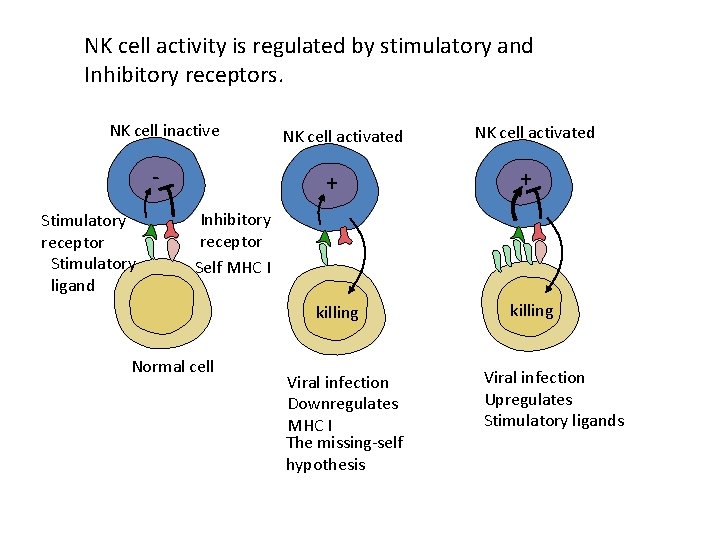
NK cell activity is regulated by stimulatory and Inhibitory receptors. NK cell inactive Stimulatory receptor Stimulatory ligand NK cell activated + Inhibitory receptor Self MHC I killing Normal cell Viral infection Downregulates MHC I The missing-self hypothesis killing Viral infection Upregulates Stimulatory ligands
![Inhibitory receptors contain ITIM immunoreceptor tyrosinebased inhibitory motif in their cytoplasmic domains IVXYXXL Y Inhibitory receptors contain ITIM (immunoreceptor tyrosine-based inhibitory motif) in their cytoplasmic domains. [I/V]XYXXL: Y](https://slidetodoc.com/presentation_image_h2/4627a2cb187d98d2a5fbe64850f0660d/image-11.jpg)
Inhibitory receptors contain ITIM (immunoreceptor tyrosine-based inhibitory motif) in their cytoplasmic domains. [I/V]XYXXL: Y is the substrate of tyrosine kinases. Phosphorylated ITIM recruits phosphatases (SHP-1) that counteract the ITIM Phosphorylation cascade of signal transduction. Stimulatory receptors contain short cytoplasmic domain without ITIM. The transmembrane domain associates with signal transduction molecules that contain ITAM (immunoreceptor Tyrosine-based activating motif) in the cytoplasmic domain. YXX[L/I]X 6 -9 YXX[L/I]: Y is the substrate of typrosine kinases. Phosphorylated ITAM recruits and activates additional kinases for signal ITAM transduction.

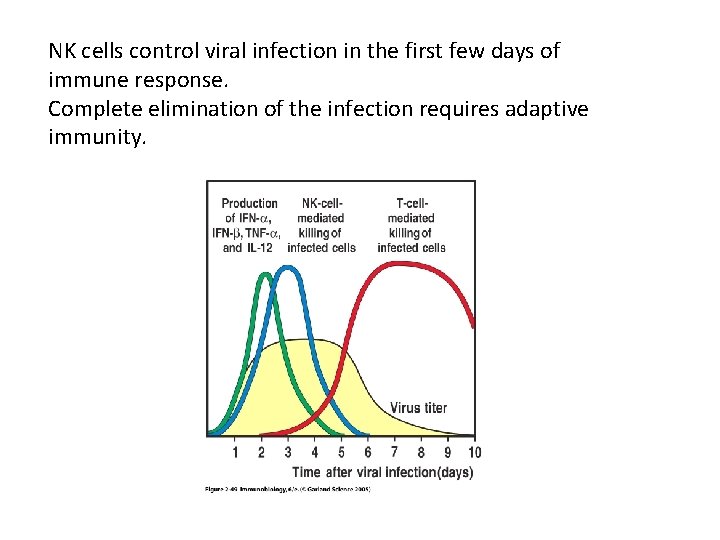
NK cells control viral infection in the first few days of immune response. Complete elimination of the infection requires adaptive immunity.

Viral countermeasures against NK cells • The presence of HIV particles in the blood alters the expression of a number of molecules on the surface of NK cells. • This reduces the efficiency of NK cell activities, including the ability to kill virus-infected cells and to secrete γ-interferon.

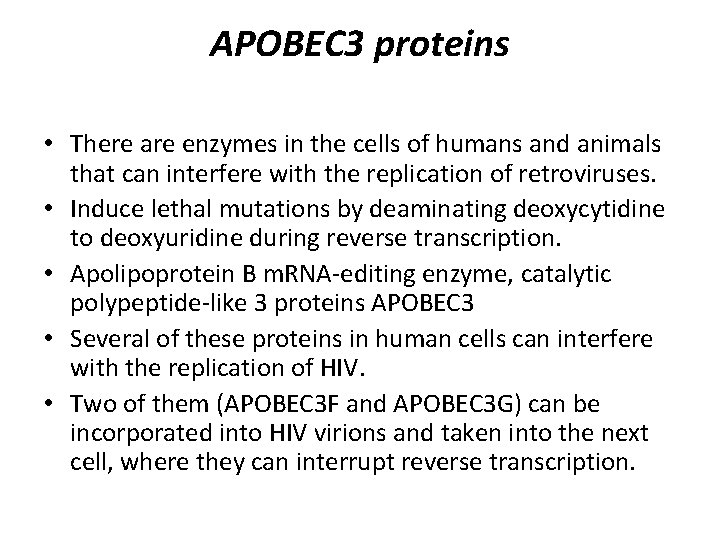
APOBEC 3 proteins • There are enzymes in the cells of humans and animals that can interfere with the replication of retroviruses. • Induce lethal mutations by deaminating deoxycytidine to deoxyuridine during reverse transcription. • Apolipoprotein B m. RNA-editing enzyme, catalytic polypeptide-like 3 proteins APOBEC 3 • Several of these proteins in human cells can interfere with the replication of HIV. • Two of them (APOBEC 3 F and APOBEC 3 G) can be incorporated into HIV virions and taken into the next cell, where they can interrupt reverse transcription.

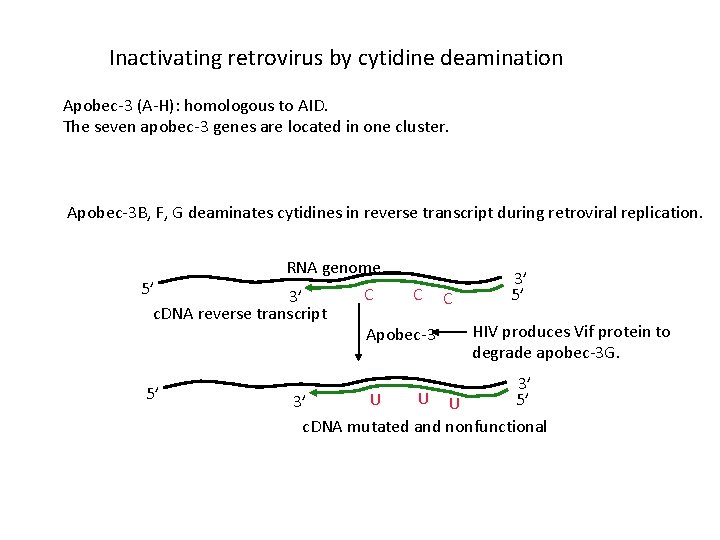
Inactivating retrovirus by cytidine deamination Apobec-3 (A-H): homologous to AID. The seven apobec-3 genes are located in one cluster. Apobec-3 B, F, G deaminates cytidines in reverse transcript during retroviral replication. RNA genome 5’ C C C 3’ c. DNA reverse transcript Apobec-3 5’ 3’ 5’ HIV produces Vif protein to degrade apobec-3 G. 3’ 5’ U U U 3’ c. DNA mutated and nonfunctional

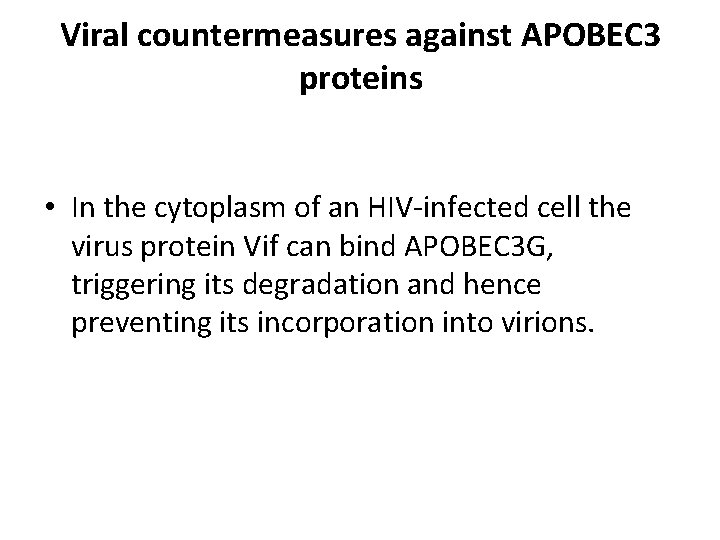
Viral countermeasures against APOBEC 3 proteins • In the cytoplasm of an HIV-infected cell the virus protein Vif can bind APOBEC 3 G, triggering its degradation and hence preventing its incorporation into virions.

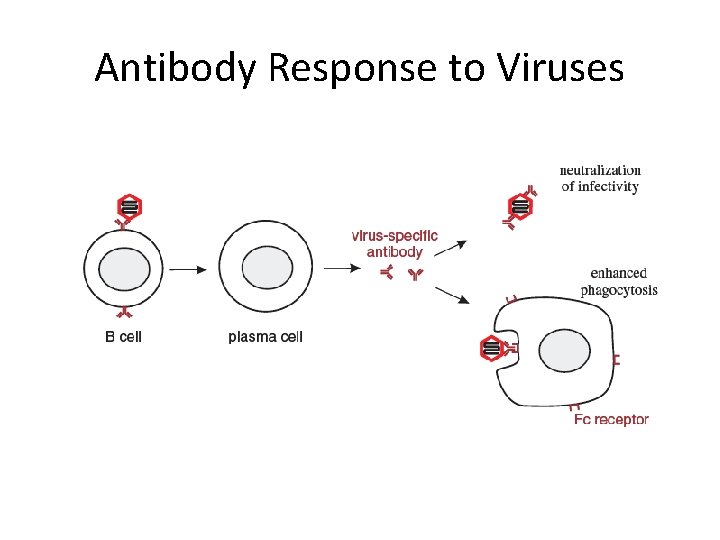
Antibody Response to Viruses

Antibody Responses • Virus-specific antibody can coat both virions and virusinfected cells, and this may lead to their destruction by a variety of mechanisms. • A number of cell types in the immune system have receptors for the Fc region of Ig. G , allowing these cells to attach to antibody-coated virions and cells. • Cell types that have Ig. G Fc receptors include neutrophils and macrophages (these cell types are phagocytes and may phagocytose the antibody-coated materials; they may also kill cells without phagocytosing them) • NK cells (these cells may kill virus-infected cells by insertion of perforins into their membranes). • Activation of complement

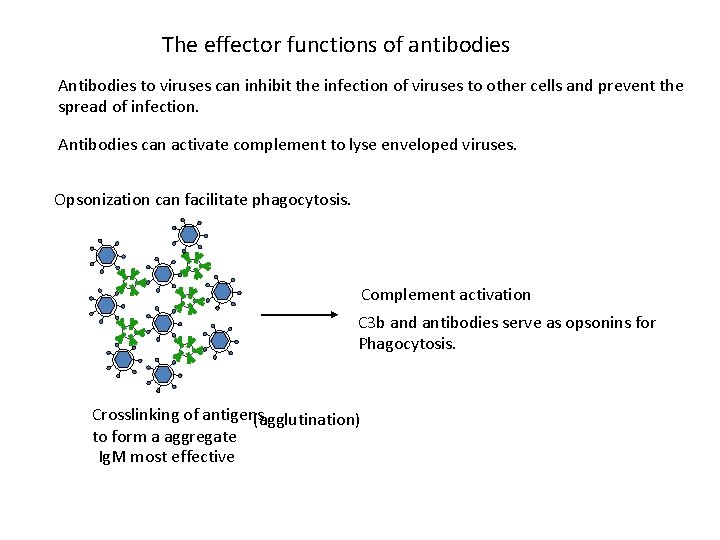
The effector functions of antibodies Antibodies to viruses can inhibit the infection of viruses to other cells and prevent the spread of infection. Antibodies can activate complement to lyse enveloped viruses. Opsonization can facilitate phagocytosis. Complement activation C 3 b and antibodies serve as opsonins for Phagocytosis. Crosslinking of antigens (agglutination) to form a aggregate Ig. M most effective

Neutralization of infectivity • Which may occur by a variety of mechanisms. • Release of nucleic acid from virions. • In studies with several viruses, including poliovirus, it was found that antibodies can attach to virions, and then detach leaving empty capsids devoid of their genomes. • Prevention of virion attachment to cell receptors - Antibody bound to a virion may mask virus attachment sites. Not all virus attachment sites, however, are accessible to antibodies; those of most picornaviruses are in deep canyons • Release of virions that have attached to cell receptors. • Inhibition of entry into the cell. Antibody coating fusion proteins on an enveloped virion may inhibit fusion of the envelope with a cell membrane. • Inhibition of genome uncoating.

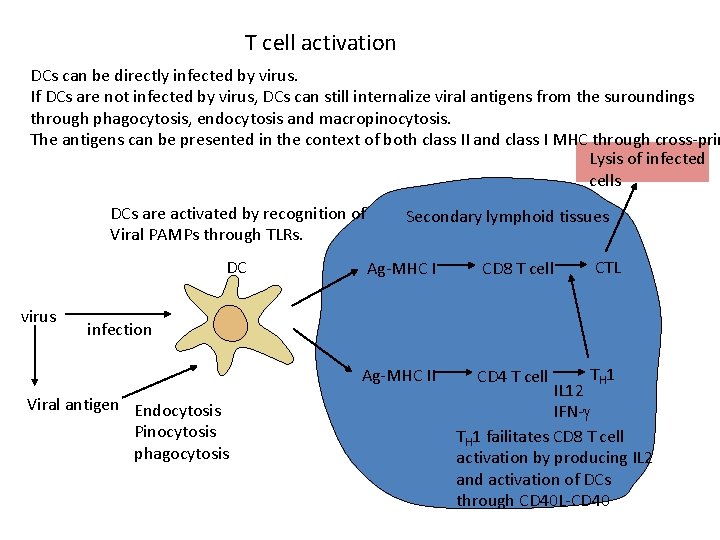
T cell activation DCs can be directly infected by virus. If DCs are not infected by virus, DCs can still internalize viral antigens from the suroundings through phagocytosis, endocytosis and macropinocytosis. The antigens can be presented in the context of both class II and class I MHC through cross-prim Lysis of infected cells DCs are activated by recognition of Viral PAMPs through TLRs. DC virus Secondary lymphoid tissues Ag-MHC I CD 8 T cell CTL Ag-MHC II CD 4 T cell TH 1 infection Viral antigen Endocytosis Pinocytosis phagocytosis IL 12 IFN- TH 1 failitates CD 8 T cell activation by producing IL 2 and activation of DCs through CD 40 L-CD 40

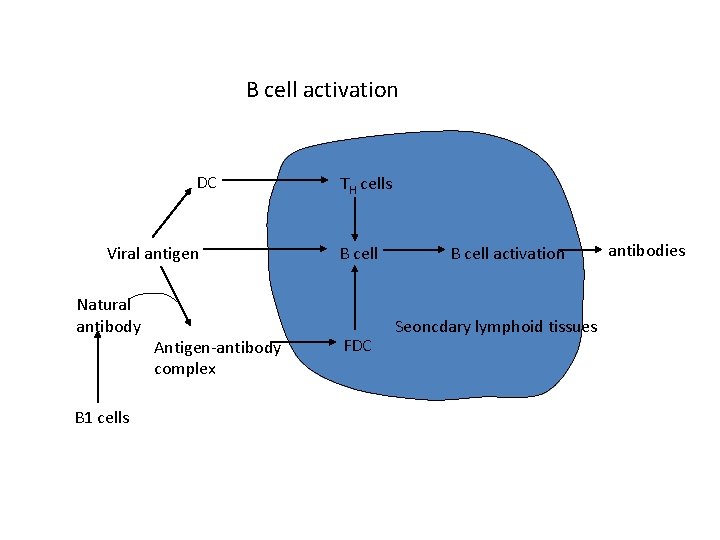
B cell activation DC Viral antigen Natural antibody B 1 cells Antigen-antibody complex TH cells B cell FDC B cell activation Seoncdary lymphoid tissues antibodies

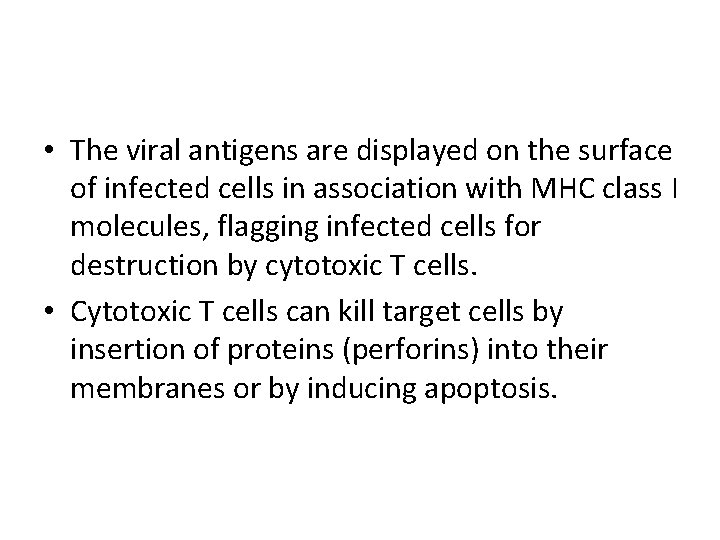
• The viral antigens are displayed on the surface of infected cells in association with MHC class I molecules, flagging infected cells for destruction by cytotoxic T cells. • Cytotoxic T cells can kill target cells by insertion of proteins (perforins) into their membranes or by inducing apoptosis.

Viral countermeasures against cytotoxic T cells • Some viruses, such as herpesviruses, reduce the level of expression of MHC class I molecules at the surface of infected cells, thereby making it more difficult for cytotoxic T cells to recognize infected cells.

Immunological memory • The quantity and quality of the adaptive immune response depends on whether or not the host is encountering the virus for the first time. • Some B cells and T cells can survive as memory cells long after the first or subsequent encounters. Memory cells have returned to a resting state, from which they can be reactivated if they encounter the same antigen again. • These cells are the basis of immunological memory, which can be formed as a result of a natural infection, but also as a result of encountering antigens in vaccines. • The outcome of infection of a vertebrate animal with a virus may depend on whether or not the host has immunological memory of the virus antigens. • If immunological memory is present then signs and symptoms of disease are likely to be less severe, or totally absent.

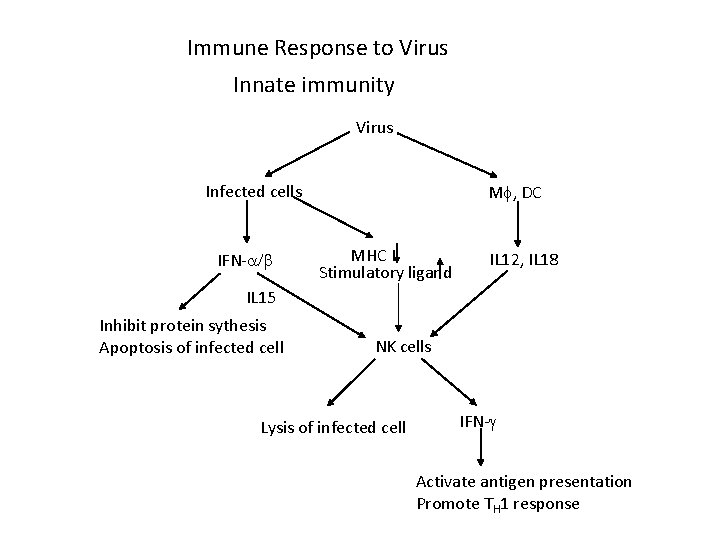
Immune Response to Virus Innate immunity Virus Infected cells IFN- M , DC MHC I Stimulatory ligand IL 12, IL 18 IL 15 Inhibit protein sythesis Apoptosis of infected cell NK cells Lysis of infected cell IFN- Activate antigen presentation Promote TH 1 response

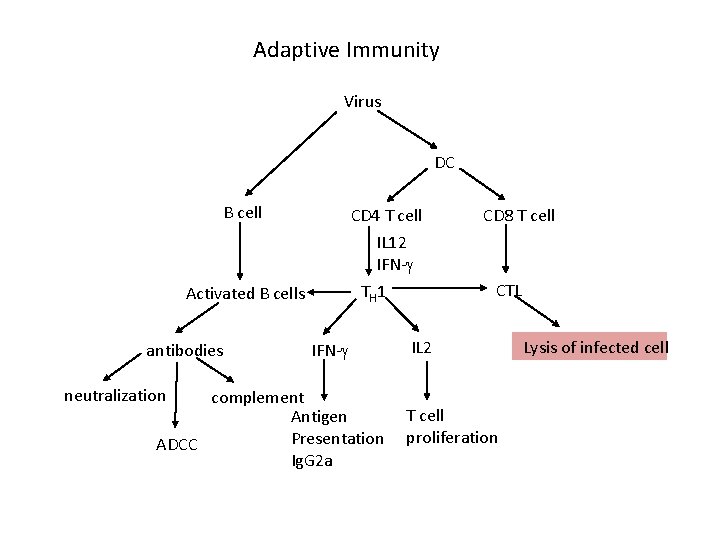
Adaptive Immunity Virus DC B cell CD 4 T cell IL 12 IFN- antibodies neutralization CTL TH 1 Activated B cells IFN- complement Antigen Presentation ADCC Ig. G 2 a CD 8 T cell IL 2 T cell proliferation Lysis of infected cell

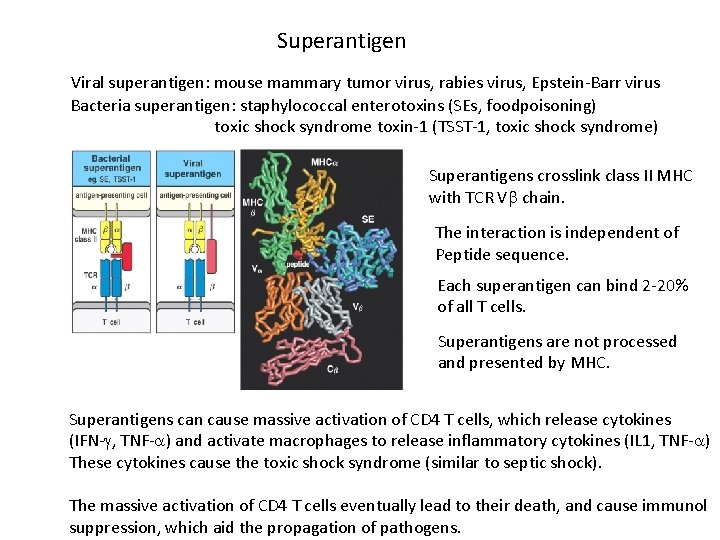
Superantigen Viral superantigen: mouse mammary tumor virus, rabies virus, Epstein-Barr virus Bacteria superantigen: staphylococcal enterotoxins (SEs, foodpoisoning) toxic shock syndrome toxin-1 (TSST-1, toxic shock syndrome) Superantigens crosslink class II MHC with TCR V chain. The interaction is independent of Peptide sequence. Each superantigen can bind 2 -20% of all T cells. Superantigens are not processed and presented by MHC. Superantigens can cause massive activation of CD 4 T cells, which release cytokines (IFN- , TNF- ) and activate macrophages to release inflammatory cytokines (IL 1, TNF- ) These cytokines cause the toxic shock syndrome (similar to septic shock). The massive activation of CD 4 T cells eventually lead to their death, and cause immunol suppression, which aid the propagation of pathogens.

RNA silencing • RNA silencing, also known as posttranscriptional gene silencing or RNA interference (RNAi), is an intracellular process that is induced by ds. RNA. • The process results in the destruction of m. RNAs that have the same sequence as the inducing ds. RNA; both cellular and viral m. RNAs can be destroyed.

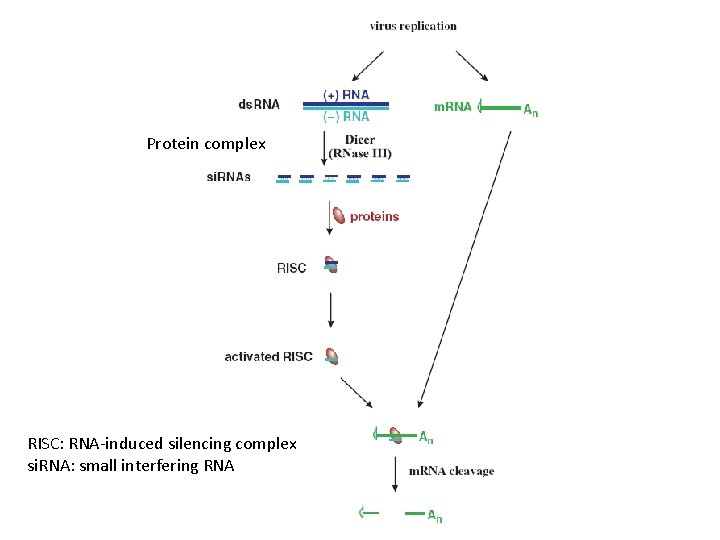
Protein complex RISC: RNA-induced silencing complex si. RNA: small interfering RNA

Viral countermeasures against RNA silencing • Some plant viruses encode proteins that can suppress RNA silencing, for example the ‘helper component proteinase’ of potyviruses and the P 19 protein of tombusviruses are strong suppressors of RNA silencing.

Programmed cell death • Virus infection of a cell may initiate a process that causes the death of the cell before progeny virus has been produced, hence preventing the spread of infection to other cells. • In animal cells this suicide mechanism is known as apoptosis. • It is triggered, not only by virus infection, but also when the lifespan of cells, such as epithelial cells, is complete. • Bacteria have developed similar mechanisms to protect the species from phage infection. The death of a host bacterium before any progeny phage has been produced protects other susceptible cells from infection. These mechanisms have been found in Escherichia coli and in many other species. • If a virus-infected cell successfully completes the process of programmed cell death then it altruistically commits suicide for the benefit of either a multicellular host or a population of unicellular hosts.

Viral countermeasures against apoptosis • Many viruses have evolved mechanisms that can suppress apoptosis at a variety of points in the process, for example several DNA viruses encode proteins related to the cell BCL-2 proteins that control apoptosis. • These viral proteins block apoptosis, resulting in the survival of host cells and the completion of virus replication cycles.
 Proteins are synthesized in
Proteins are synthesized in Primary immune response and secondary immune response
Primary immune response and secondary immune response Cox 2 inhibitors
Cox 2 inhibitors Synthesized method for theory evaluation
Synthesized method for theory evaluation Bone and joint infections
Bone and joint infections Opportunistic infections
Opportunistic infections Innate immunity first line of defense
Innate immunity first line of defense Johnson and johnson botnet infections
Johnson and johnson botnet infections Retroviruses and opportunistic infections
Retroviruses and opportunistic infections Neurosiphyllis
Neurosiphyllis Can methotrexate cause yeast infections
Can methotrexate cause yeast infections Acute gingival infections
Acute gingival infections Storch infections
Storch infections Eye infections
Eye infections Genital infections
Genital infections Opportunistic infections
Opportunistic infections Storch infections
Storch infections Postpartum infections
Postpartum infections Genital infections
Genital infections A bacterial std that usually affects mucous membranes
A bacterial std that usually affects mucous membranes Replicação viral ciclo lítico e lisogênico
Replicação viral ciclo lítico e lisogênico Eline's viral
Eline's viral Dha mcq
Dha mcq Hgado
Hgado Viral
Viral Viral inoculation in embryonated egg
Viral inoculation in embryonated egg Viral life cycle
Viral life cycle Streptococcus
Streptococcus Morfologia viral
Morfologia viral Causes of viral hemorrhagic fever
Causes of viral hemorrhagic fever Section 24-1 viral structure and replication
Section 24-1 viral structure and replication Viral communications
Viral communications Viral entry
Viral entry Csf findings in meningitis table
Csf findings in meningitis table