III Writing Naming Ionic Formulas A Ionic Compound





- Slides: 16

III. Writing & Naming Ionic Formulas

A. Ionic Compound Basics �Always composed of a metal and a nonmetal �Metal donates an electron to the nonmetal �Metal becomes positively charged �Nonmetal becomes negatively charged �Opposite charges is what causes the atoms to bond to one another

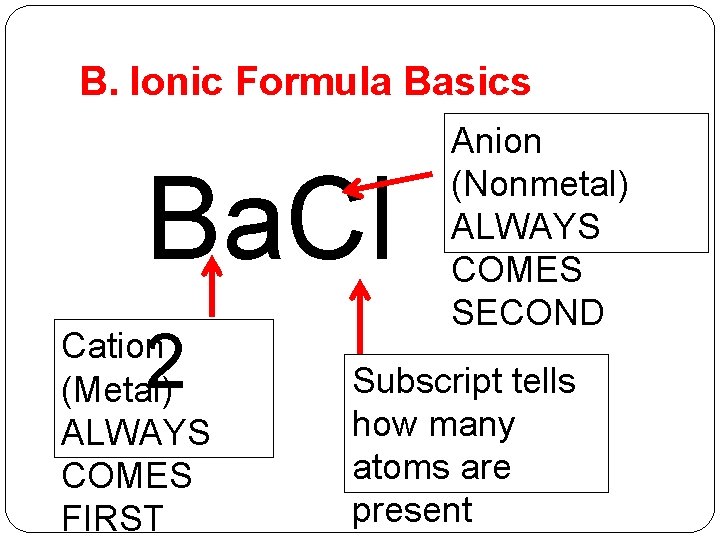
B. Ionic Formula Basics Ba. Cl 2 Cation (Metal) ALWAYS COMES FIRST Anion (Nonmetal) ALWAYS COMES SECOND Subscript tells how many atoms are present

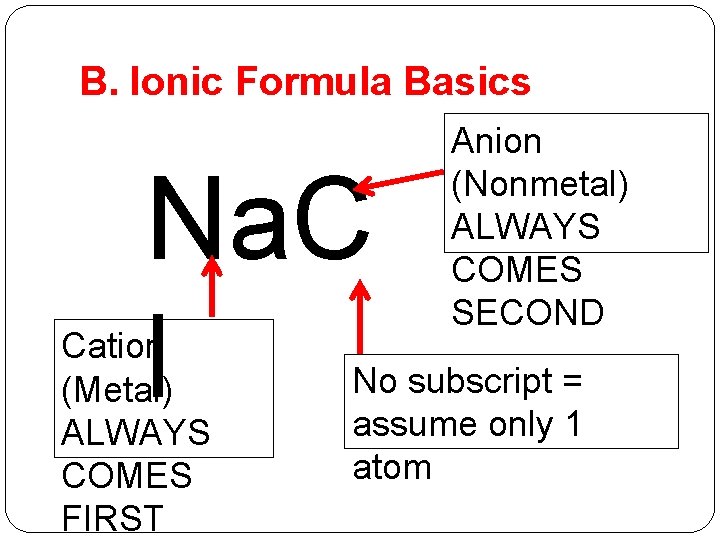
B. Ionic Formula Basics Na. C l Cation (Metal) ALWAYS COMES FIRST Anion (Nonmetal) ALWAYS COMES SECOND No subscript = assume only 1 atom

C. Writing Ionic Formulas �cations and anions combine to form NEUTRAL compounds �Positive and negative charges must cancel out when forming compounds �RULES 1. Write the symbols and charges for both the cation and anion involved 2. If both charges cancel out, simply combine to form compound 3. If charges DO NOT cancel out, add subscripts so that charges cancel

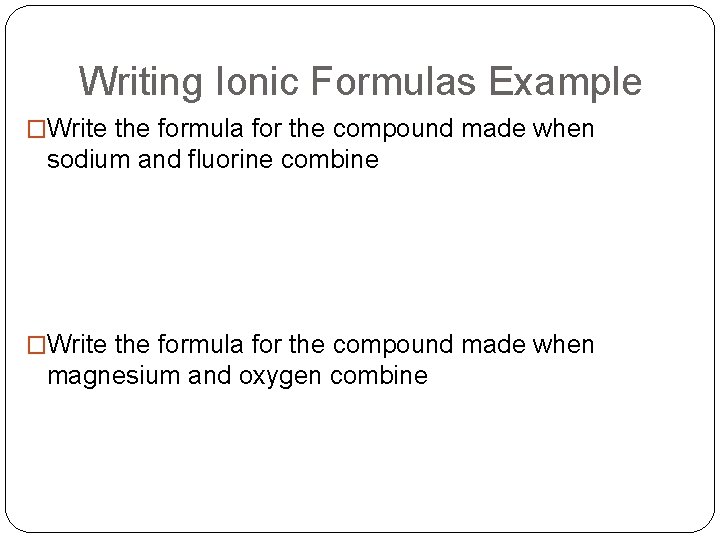
Writing Ionic Formulas Example �Write the formula for the compound made when sodium and fluorine combine �Write the formula for the compound made when magnesium and oxygen combine

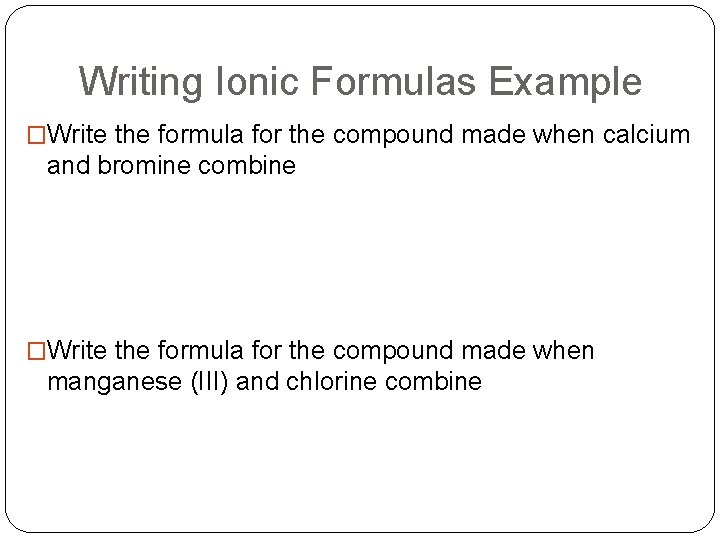
Writing Ionic Formulas Example �Write the formula for the compound made when calcium and bromine combine �Write the formula for the compound made when manganese (III) and chlorine combine

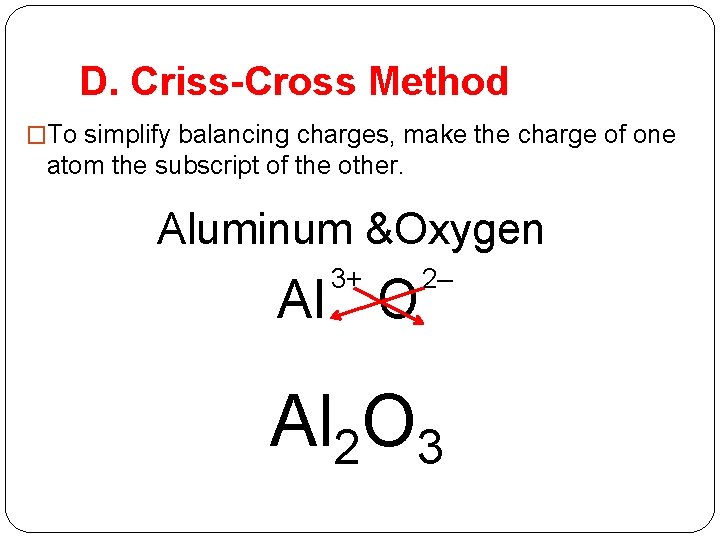
D. Criss-Cross Method �To simplify balancing charges, make the charge of one atom the subscript of the other. Aluminum &Oxygen 3+ Al O 2– Al 2 O 3

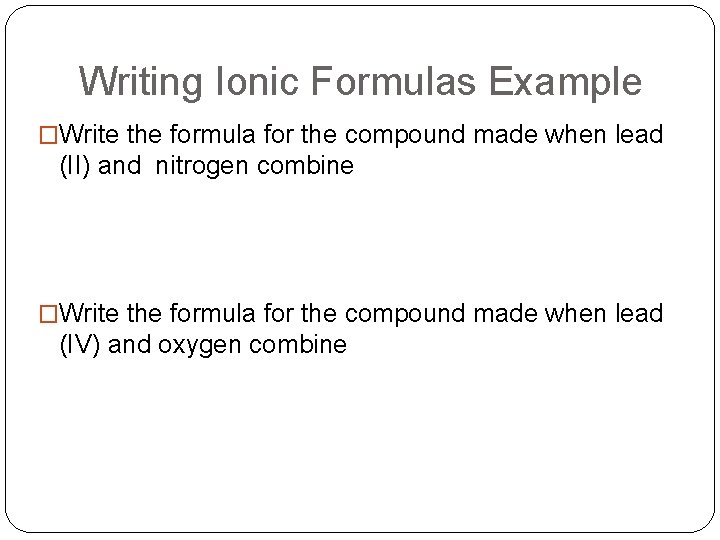
Writing Ionic Formulas Example �Write the formula for the compound made when lead (II) and nitrogen combine �Write the formula for the compound made when lead (IV) and oxygen combine

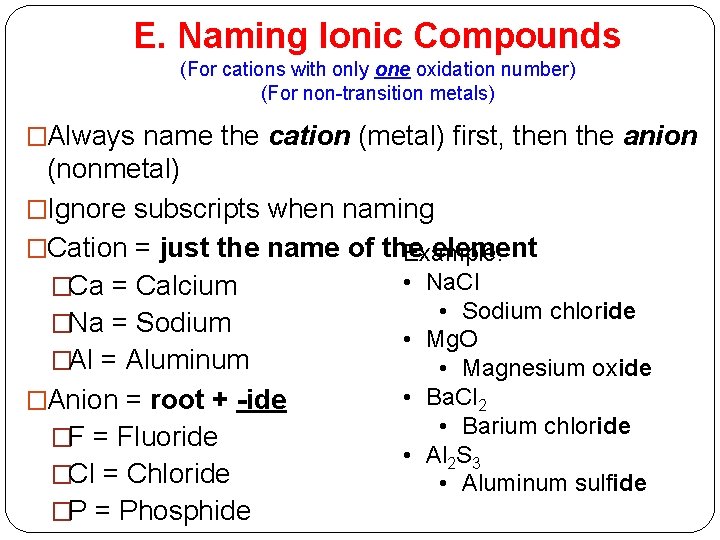
E. Naming Ionic Compounds (For cations with only one oxidation number) (For non-transition metals) �Always name the cation (metal) first, then the anion (nonmetal) �Ignore subscripts when naming �Cation = just the name of the element Example: • Na. Cl �Ca = Calcium • Sodium chloride �Na = Sodium • Mg. O �Al = Aluminum • Magnesium oxide • Ba. Cl 2 �Anion = root + -ide • Barium chloride �F = Fluoride • Al 2 S 3 �Cl = Chloride • Aluminum sulfide �P = Phosphide

Practice �Write the names for the following ionic compounds 1. Ca. Br 2 2. K 3 P 3. Be. O 4. Li. F 5. Sr. I 2

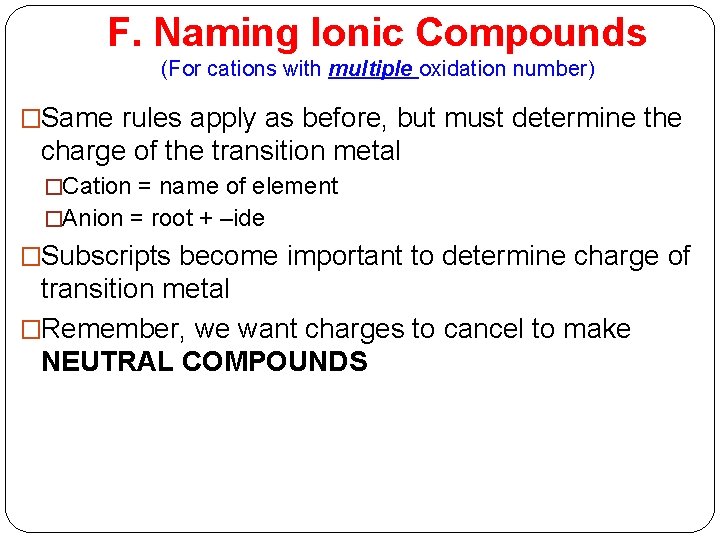
F. Naming Ionic Compounds (For cations with multiple oxidation number) �Same rules apply as before, but must determine the charge of the transition metal �Cation = name of element �Anion = root + –ide �Subscripts become important to determine charge of transition metal �Remember, we want charges to cancel to make NEUTRAL COMPOUNDS

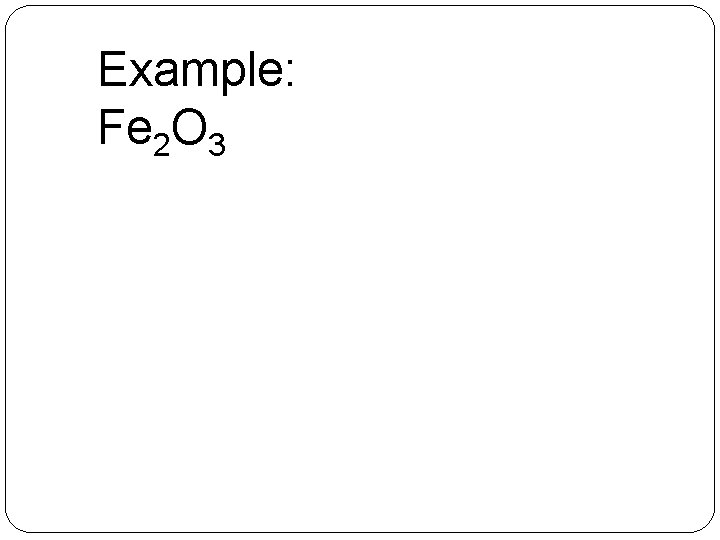
Example: Fe 2 O 3 � 1. Recognize that iron is located in the d-block (transition metal) �Therefore, does not have a “set in stone” charge �Oxygen, however, does � 2. Determine the charge of oxygen �– 2 �Since there are 3 oxygen atoms, total charge from oxygen = – 6 � 3. Determine charge of iron needed to cancel charge of oxygen �There are 2 iron atoms, therefore, each must have a +3

Example: Fe 2 O 3

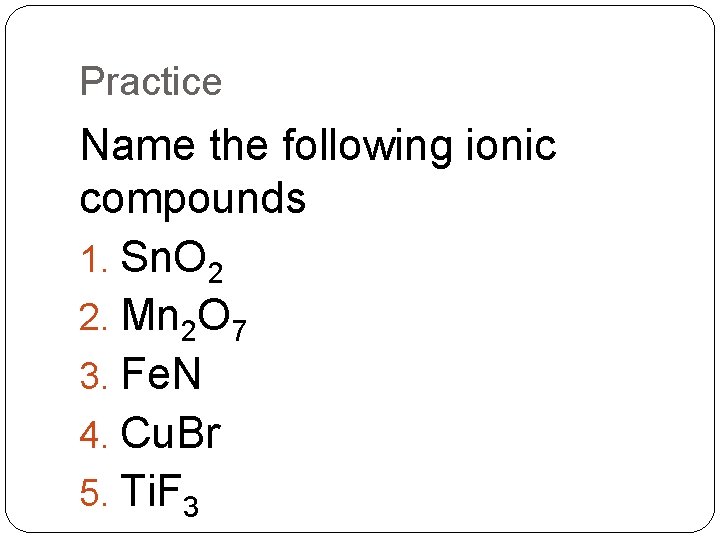
Practice Name the following ionic compounds 1. Sn. O 2 2. Mn 2 O 7 3. Fe. N 4. Cu. Br 5. Ti. F 3

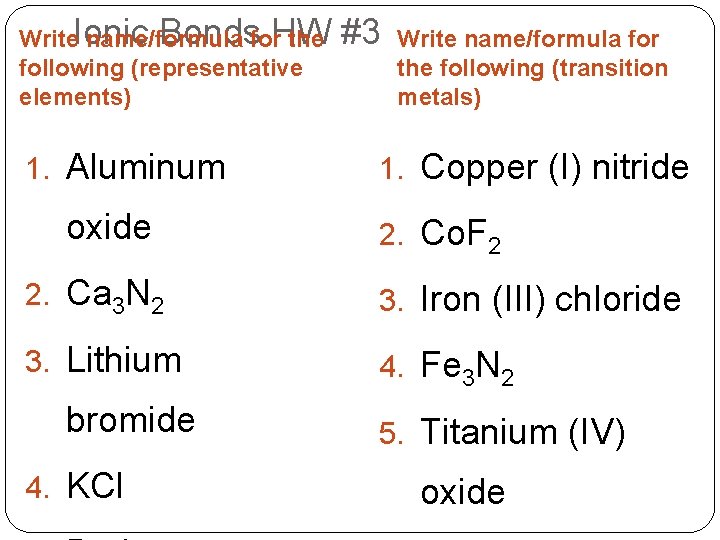
Bondsfor. HW Write. Ionic name/formula the following (representative elements) 1. Aluminum oxide #3 Write name/formula for the following (transition metals) 1. Copper (I) nitride 2. Co. F 2 2. Ca 3 N 2 3. Iron (III) chloride 3. Lithium 4. Fe 3 N 2 bromide 4. KCl 5. Titanium (IV) oxide