ICSK after Primary PCI Intracoronary Streptokinase after Primary




- Slides: 35

ICSK after Primary PCI Intracoronary Streptokinase after Primary Percutaneous Coronary Intervention Murat Sezer, Hüseyin Oflaz, Taner Gören, Irem Okcular, Berrin Umman, Yılmaz Nişancı, Ahmet Kaya Bilge, Yasemin Şanlı, Mehmet Meriç, Sabahattin Umman Istanbul University, Istanbul Faculty of Medicine, Department of Cardiology Clinical Trial Results. org

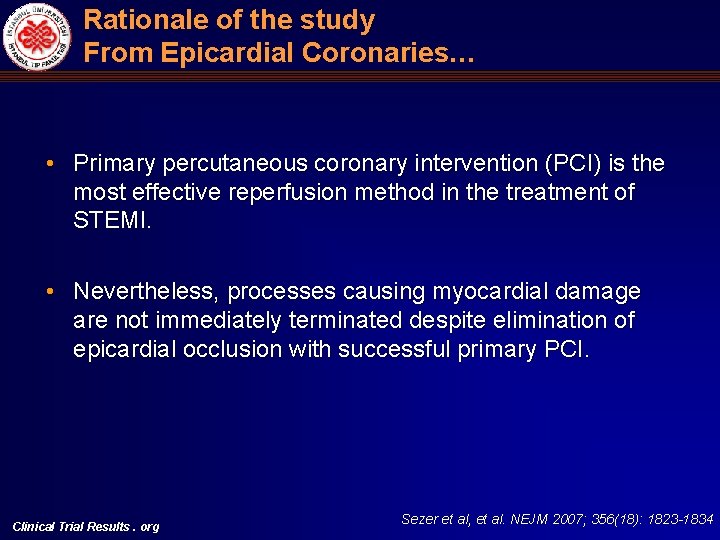
Rationale of the study From Epicardial Coronaries… • Primary percutaneous coronary intervention (PCI) is the most effective reperfusion method in the treatment of STEMI. • Nevertheless, processes causing myocardial damage are not immediately terminated despite elimination of epicardial occlusion with successful primary PCI. Clinical Trial Results. org Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

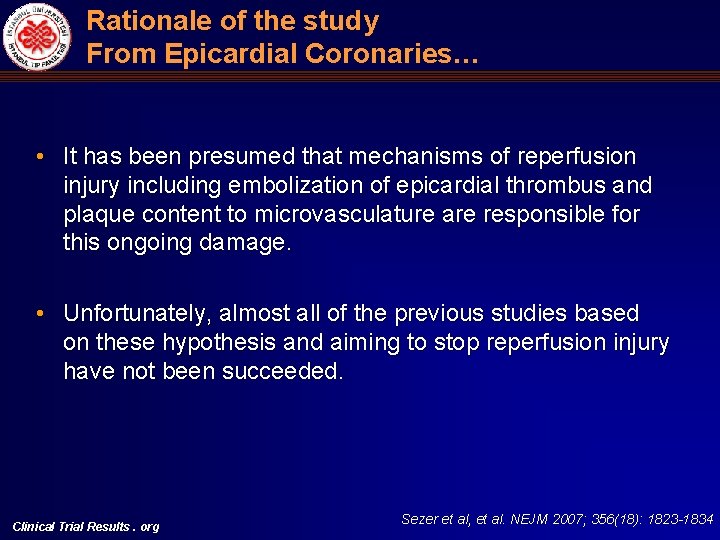
Rationale of the study From Epicardial Coronaries… • It has been presumed that mechanisms of reperfusion injury including embolization of epicardial thrombus and plaque content to microvasculature are responsible for this ongoing damage. • Unfortunately, almost all of the previous studies based on these hypothesis and aiming to stop reperfusion injury have not been succeeded. Clinical Trial Results. org Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

…to the Microvasculature • There is a growing evidence pointing concordance between myocardial and microvascular damage in STEMI patients. • Microvascular damage might not be only an accompanying process to the myocardial damage. Also there might be a causal relationship in between. • If one focused to microvasculature during peri-PCI procedure, it can be easily realized that another and important contributor might have a role in this process. insitu microvascular thrombus! Clinical Trial Results. org Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

Virchow’s Triad and Autochthonous Thrombus • All three components of Virchow’s triad (blood constituent, endothelial damage and stasis) exist at extreme levels in the microvasculature of infarcted myocardium. • Therefore, in situ (de-novo) formed fraction may constitute the main part of the thrombus located in infarct site’s microvasculature. Virchow RR. Cellular Pathology. London, Churchill, 1860. Clinical Trial Results. org Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

Coronary Vascular Resistance • If epicardial and microvascular vessels are considered as serially connected resistances, elimination of the proximal epicardial occlusion and retrieval of whole epicardial thrombus would not be enough to normalize total coronary resistance and perfusion at affected segments. Clinical Trial Results. org Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

Hypothesis • Complementary intracoronary streptokinase (ICSK) infusion immediately following primary PCI may further improve tissue level perfusion by dissolving thrombus (either in situ formed or embolized from the proximal origin) at microvascular level. • To this end, the effect of low-dose (250 k. U) ICSK, administered immediately after primary PCI, on myocardial perfusion was investigated prospectively. Clinical Trial Results. org Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

Inclusion/Exclusion Criteria • Inclusion criteria: – – – Ongoing chest pain, ST segment elevation on electrocardiogram, Occlusion of the infarct related artery at angiography (Thrombolysis in Myocardial Infarction [TIMI] 0 I flow) • Exclusion criteria: – – – Culprit lesion in a saphenous vein graft, Additional narrowing >50% distal to the culprit lesion, Left bundle branch block, History of prior myocardial infarction, and Contraindications to streptokinase, tirofiban, aspirin, clopidogrel or heparin. Clinical Trial Results. org Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

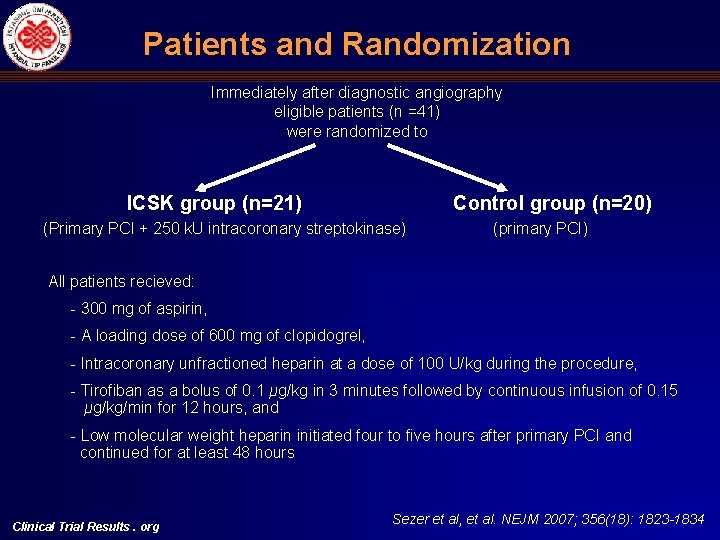
Patients and Randomization Immediately after diagnostic angiography eligible patients (n =41) were randomized to ICSK group (n=21) Control group (n=20) (Primary PCI + 250 k. U intracoronary streptokinase) (primary PCI) All patients recieved: - 300 mg of aspirin, - A loading dose of 600 mg of clopidogrel, - Intracoronary unfractioned heparin at a dose of 100 U/kg during the procedure, - Tirofiban as a bolus of 0. 1 μg/kg in 3 minutes followed by continuous infusion of 0. 15 μg/kg/min for 12 hours, and - Low molecular weight heparin initiated four to five hours after primary PCI and continued for at least 48 hours Clinical Trial Results. org Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

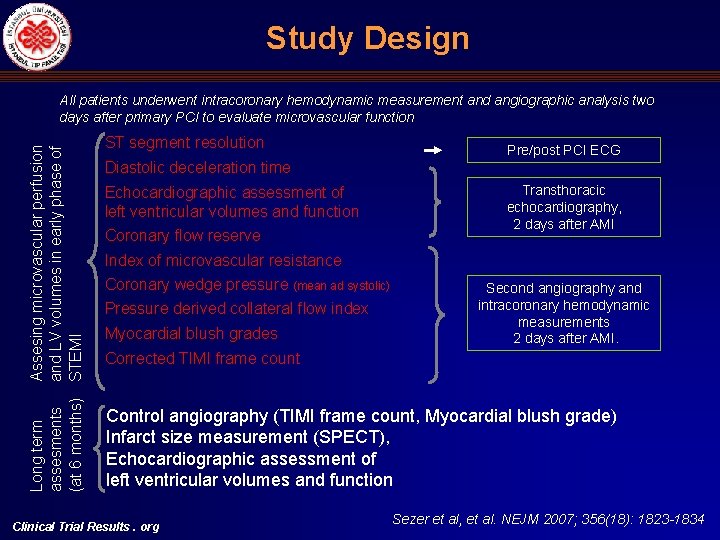
Study Design Long term Assesing microvascular perfusion assesments and LV volumes in early phase of (at 6 months) STEMI All patients underwent intracoronary hemodynamic measurement and angiographic analysis two days after primary PCI to evaluate microvascular function ST segment resolution Pre/post PCI ECG Diastolic deceleration time Echocardiographic assessment of left ventricular volumes and function Coronary flow reserve Index of microvascular resistance Coronary wedge pressure (mean ad systolic) Pressure derived collateral flow index Myocardial blush grades Transthoracic echocardiography, 2 days after AMI Second angiography and intracoronary hemodynamic measurements 2 days after AMI. Corrected TIMI frame count Control angiography (TIMI frame count, Myocardial blush grade) Infarct size measurement (SPECT), Echocardiographic assessment of left ventricular volumes and function Clinical Trial Results. org Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

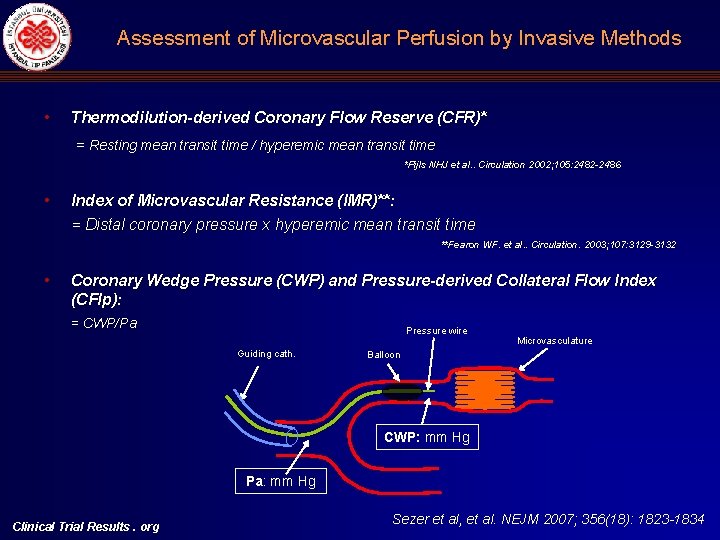
Assessment of Microvascular Perfusion by Invasive Methods • Thermodilution-derived Coronary Flow Reserve (CFR)* = Resting mean transit time / hyperemic mean transit time *Pijls NHJ et al. . Circulation 2002; 105: 2482 -2486 • Index of Microvascular Resistance (IMR)**: = Distal coronary pressure x hyperemic mean transit time **Fearon WF. et al. . Circulation. 2003; 107: 3129 -3132 • Coronary Wedge Pressure (CWP) and Pressure-derived Collateral Flow Index (CFIp): = CWP/Pa Pressure wire Guiding cath. Microvasculature Balloon CWP: mm Hg Pa: mm Hg Clinical Trial Results. org Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

Primary and Secondary Endpoints Primary Endpoints: • All three components of Virchow’s triad (blood constituent, endothelial damage and stasis) exist at extreme levels in the microvasculature of infarcted myocardium. • Coronary flow reserve • Index of microvascular resistance • Coronary wedge pressure • Collateral flow index • Coronary diastolic deceleration time Secondary Endpoints: • Corrected TIMI frame count • Myocardial blush grade • Infarct size • Changes in left ventricular volumes • Major adverse cardiac events (reinfarction, revascularization and death) Clinical Trial Results. org Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

Statistical Analysis • Calculations were then made of the necessary sample size to detect a 30% difference between the intracoronary streptokinase and control groups for each end point (α=0. 05, β=0. 20, power=0. 80). • Group proportions were compared by means of the chisquare test or Fisher exact test, as appropriate. Group means were compared by Student’s t test for independent groups or the Mann-Whitney U test, for variables with normal or non-normal distribution, respectively Clinical Trial Results. org Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

Statistical Analysis (cont. ) • Group means were also adjusted for possible confounding factors (age, pain-to-balloon time, diabetes, hypertension, hyperlipidemia, pre-myocardial infarction angina, slow flow, side branch embolization, smoking and infarct location) using analysis of covariance (ANCOVA). Clinical Trial Results. org Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

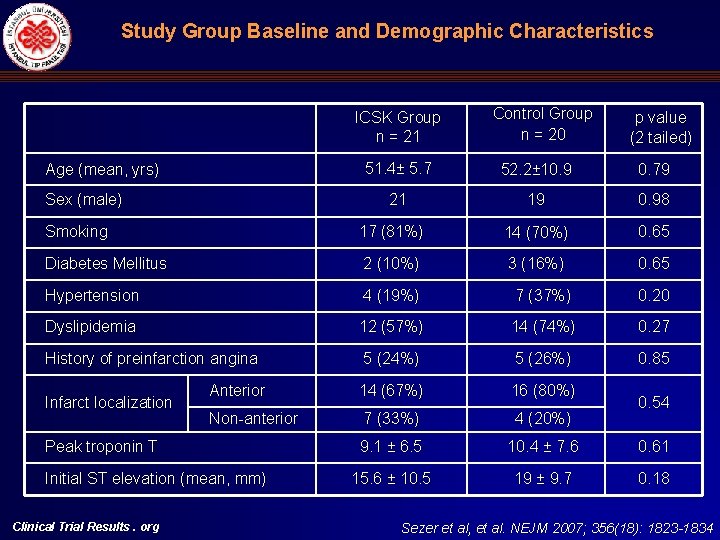
Study Group Baseline and Demographic Characteristics ICSK Group n = 21 Age (mean, yrs) Sex (male) Control Group n = 20 p value (2 tailed) 51. 4± 5. 7 52. 2± 10. 9 0. 79 21 19 0. 98 Smoking 17 (81%) 14 (70%) 0. 65 Diabetes Mellitus 2 (10%) 3 (16%) 0. 65 Hypertension 4 (19%) 7 (37%) 0. 20 Dyslipidemia 12 (57%) 14 (74%) 0. 27 History of preinfarction angina 5 (24%) 5 (26%) 0. 85 Anterior 14 (67%) 16 (80%) Non-anterior 7 (33%) 4 (20%) 9. 1 ± 6. 5 10. 4 ± 7. 6 0. 61 15. 6 ± 10. 5 19 ± 9. 7 0. 18 Infarct localization Peak troponin T Initial ST elevation (mean, mm) Clinical Trial Results. org 0. 54 Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

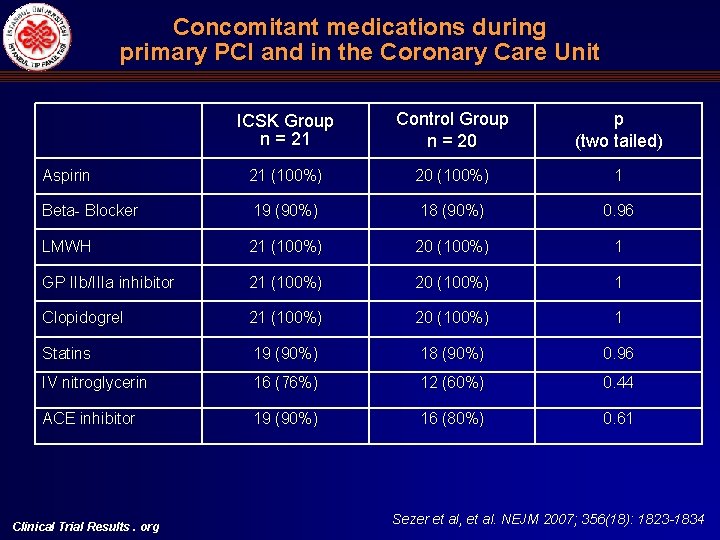
Concomitant medications during primary PCI and in the Coronary Care Unit ICSK Group n = 21 Control Group n = 20 p (two tailed) Aspirin 21 (100%) 20 (100%) 1 Beta- Blocker 19 (90%) 18 (90%) 0. 96 LMWH 21 (100%) 20 (100%) 1 GP IIb/IIIa inhibitor 21 (100%) 20 (100%) 1 Clopidogrel 21 (100%) 20 (100%) 1 Statins 19 (90%) 18 (90%) 0. 96 IV nitroglycerin 16 (76%) 12 (60%) 0. 44 ACE inhibitor 19 (90%) 16 (80%) 0. 61 Clinical Trial Results. org Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

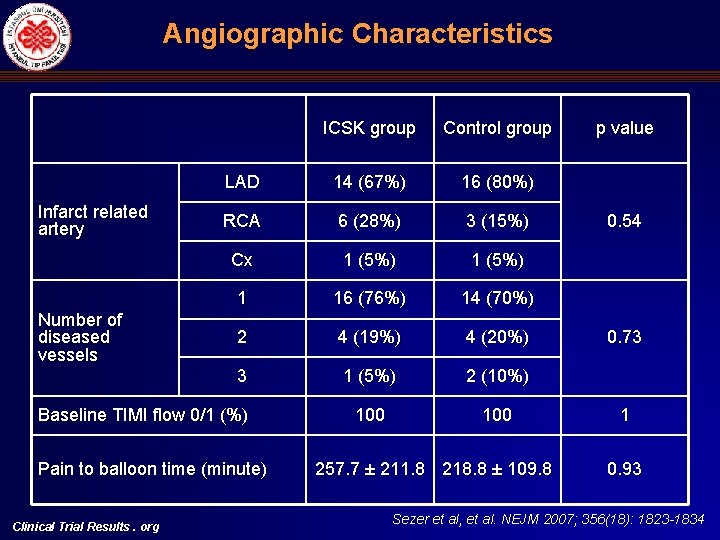
Angiographic Characteristics Infarct related artery Number of diseased vessels ICSK group Control group LAD 14 (67%) 16 (80%) RCA 6 (28%) 3 (15%) Cx 1 (5%) 1 16 (76%) 14 (70%) 2 4 (19%) 4 (20%) 3 1 (5%) 2 (10%) 100 Baseline TIMI flow 0/1 (%) Pain to balloon time (minute) Clinical Trial Results. org 257. 7 ± 211. 8 218. 8 ± 109. 8 p value 0. 54 0. 73 1 0. 93 Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

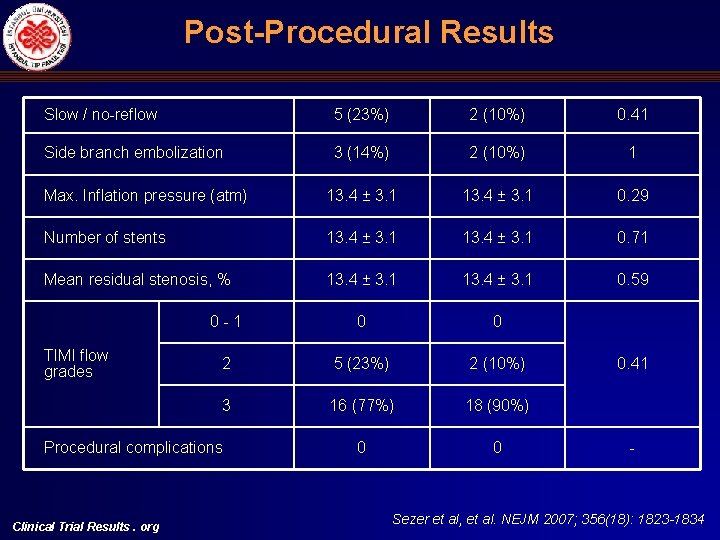
Post Procedural Results Slow / no-reflow 5 (23%) 2 (10%) 0. 41 Side branch embolization 3 (14%) 2 (10%) 1 Max. Inflation pressure (atm) 13. 4 ± 3. 1 0. 29 Number of stents 13. 4 ± 3. 1 0. 71 Mean residual stenosis, % 13. 4 ± 3. 1 0. 59 0 -1 0 0 2 5 (23%) 2 (10%) 3 16 (77%) 18 (90%) 0 0 TIMI flow grades Procedural complications Clinical Trial Results. org 0. 41 - Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

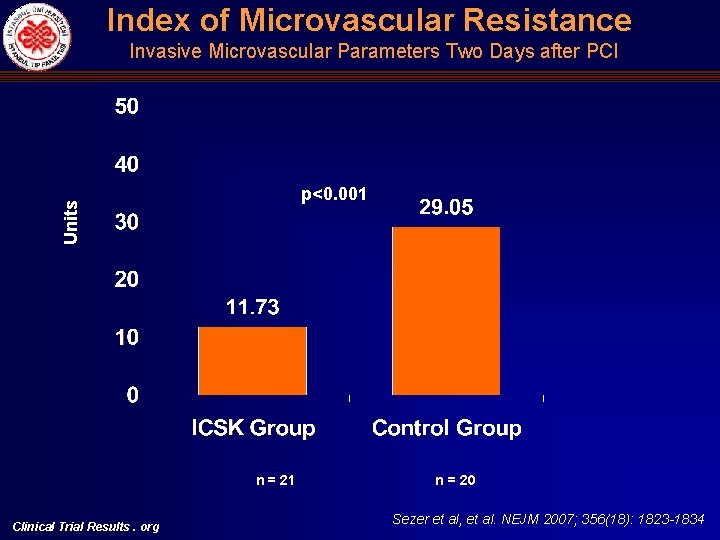
Index of Microvascular Resistance Invasive Microvascular Parameters Two Days after PCI Units p<0. 001 n = 21 Clinical Trial Results. org n = 20 Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

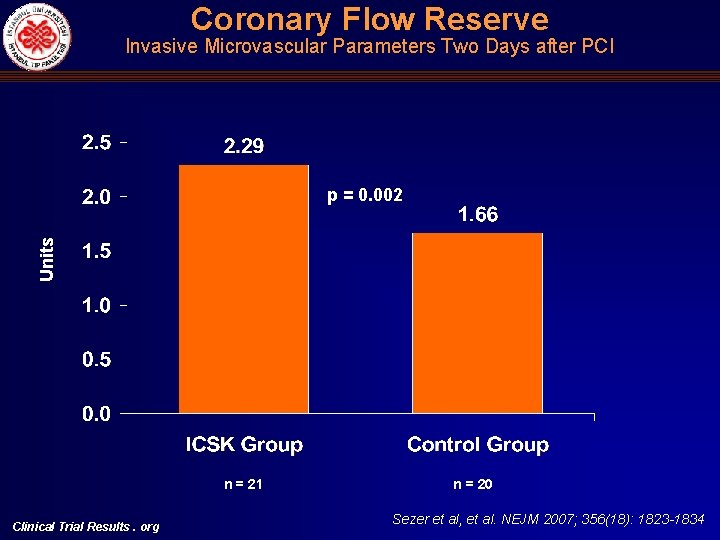
Coronary Flow Reserve Invasive Microvascular Parameters Two Days after PCI Units p = 0. 002 n = 21 Clinical Trial Results. org n = 20 Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

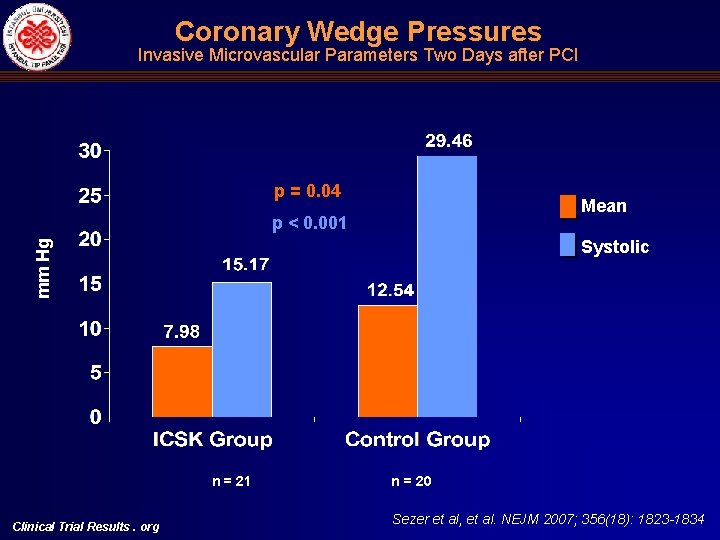
Coronary Wedge Pressures Invasive Microvascular Parameters Two Days after PCI p = 0. 04 Mean p < 0. 001 mm Hg Systolic n = 21 Clinical Trial Results. org n = 20 Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

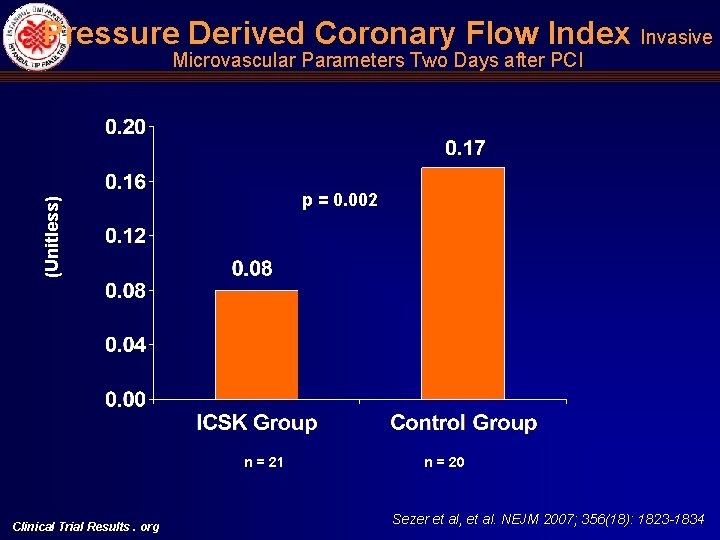
Pressure Derived Coronary Flow Index Invasive Microvascular Parameters Two Days after PCI (Unitless) p = 0. 002 n = 21 Clinical Trial Results. org n = 20 Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

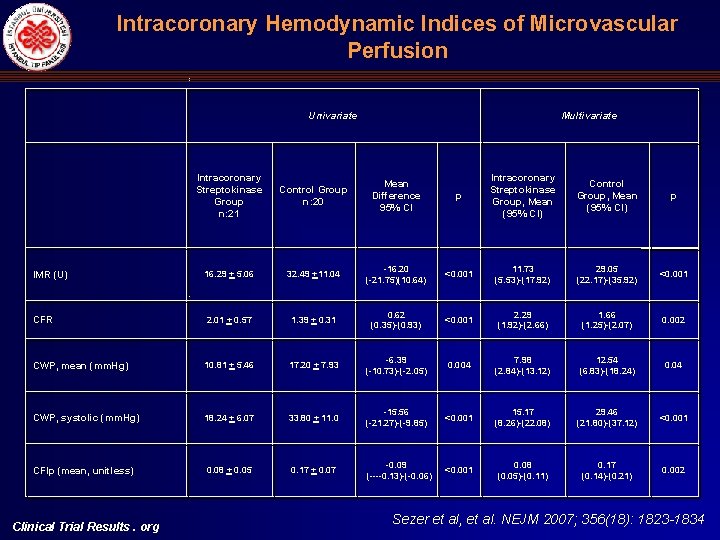
Intracoronary Hemodynamic Indices of Microvascular Perfusion Univariate Multivariate Intracoronary Streptokinase Group n: 21 Control Group n: 20 Mean Difference 95% CI p Intracoronary Streptokinase Group, Mean (95% CI) Control Group, Mean (95% CI) p IMR (U) 16. 29 + 5. 06 32. 49 +11. 04 16. 20 ( 21. 75)(10. 64) <0. 001 11. 73 (5. 53) (17. 92) 29. 05 (22. 17) (35. 92) <0. 001 CFR 2. 01 + 0. 57 1. 39 + 0. 31 0. 62 (0. 35) (0. 93) <0. 001 2. 29 (1. 92) (2. 66) 1. 66 (1. 25) (2. 07) 0. 002 CWP, mean (mm. Hg) 10. 81 + 5. 46 17. 20 + 7. 93 6. 39 ( 10. 73) ( 2. 05) 0. 004 7. 98 (2. 84) (13. 12) 12. 54 (6. 83) (18. 24) 0. 04 CWP, systolic (mm. Hg) 18. 24 + 6. 07 33. 80 + 11. 0 15. 56 ( 21. 27) ( 9. 85) <0. 001 15. 17 (8. 26) (22. 08) 29. 46 (21. 80) (37. 12) <0. 001 CFIp (mean, unitless) 0. 08 + 0. 05 0. 17 + 0. 07 0. 09 ( 0. 13) ( 0. 06) <0. 001 0. 08 (0. 05) (0. 11) 0. 17 (0. 14) (0. 21) 0. 002 Clinical Trial Results. org Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

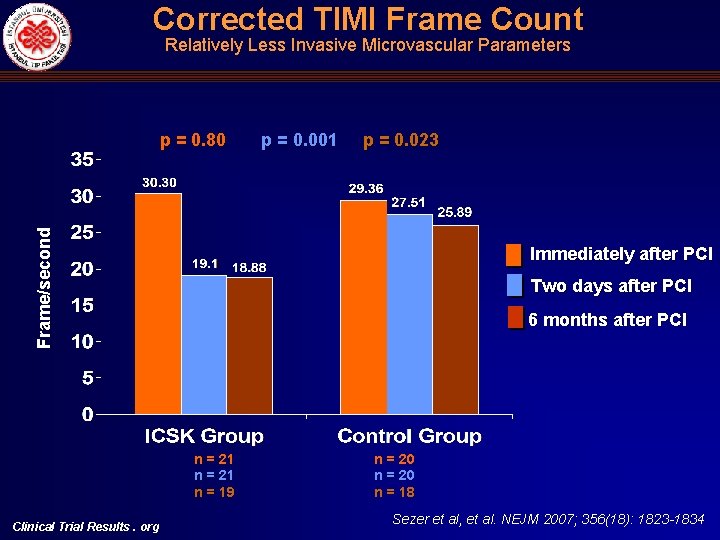
Corrected TIMI Frame Count Relatively Less Invasive Microvascular Parameters p = 0. 001 p = 0. 023 Frame/second p = 0. 80 Immediately after PCI Two days after PCI 6 months after PCI n = 21 n = 19 Clinical Trial Results. org n = 20 n = 18 Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

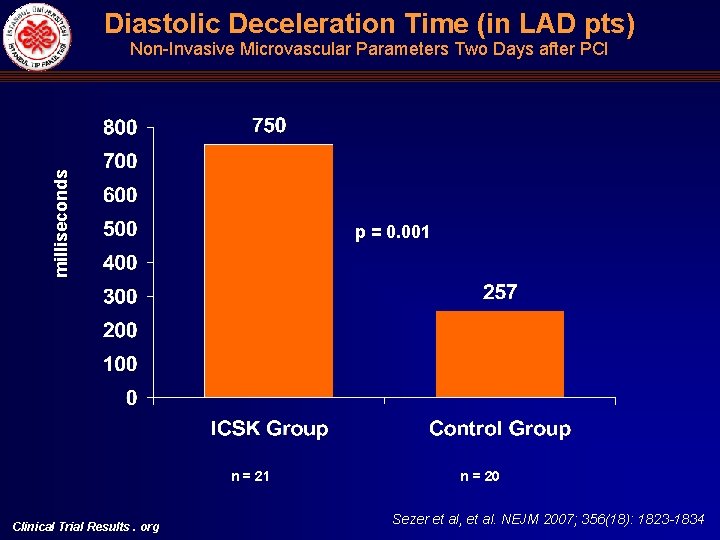
Diastolic Deceleration Time (in LAD pts) milliseconds Non-Invasive Microvascular Parameters Two Days after PCI p = 0. 001 n = 21 Clinical Trial Results. org n = 20 Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

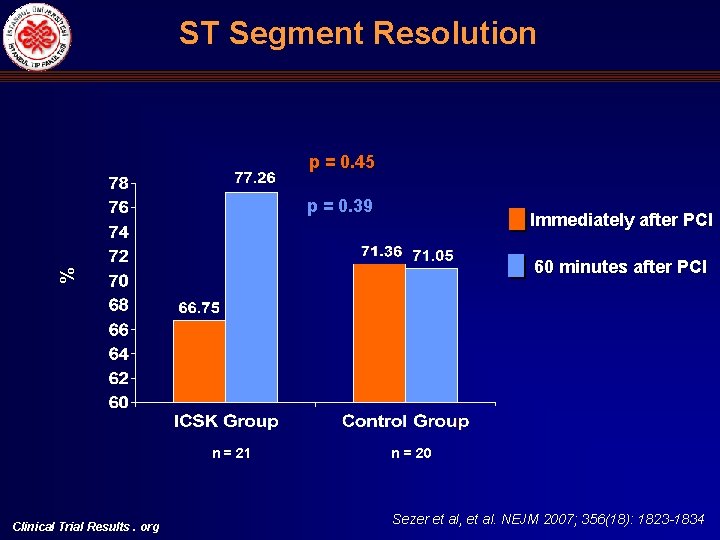
ST Segment Resolution p = 0. 45 p = 0. 39 Immediately after PCI % 60 minutes after PCI n = 21 Clinical Trial Results. org n = 20 Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

Angiographic (c. TFC, MBG), Electrocardiographic (STR) and Echocardiographic (DDT) Indices of Microvascular Perfusion Univariate ICSK group c. TFC mean Control Mean diff. p ICSK group Control p Immediately after primary PCI 33. 6 + 9. 45 34. 44 + 8. 26 -0. 79 (-6. 66)-(5. 08) 0. 69 30. 30 (23. 14)-(37. 46) 29. 36 (21. 48)-(37. 25) 0. 80 Two days after primary PCI 22. 52 + 5. 58 31. 79 + 7. 58 -9. 27 (-13. 50)-(-5. 03) <0. 001 19. 10 (14. 16)-(24. 04) 27. 51 (22. 03)-(32. 99) 0. 001 Six months after primary PCI 21. 42 + 4. 98 27. 62 + 6. 46 -6. 2 (-11. 00)-(-1. 39) 0. 014 18. 88 (13. 57)-(24. 18) 25. 89 (18. 76)-(33. 02) 0. 023 10 (50%) 13 (72%) - - - Immediately after primary PCI MBG Multivariate Two days after primary PCI Six months after primary PCI 0/1 0. 16 0. 70 2/3 10 (50%) 5 (28%) - - - 0/1 6 (29%) 13 (68%) - - - 2/3 15 (71%) 6 (32%) - - - 0/1 1 (8. 3) 6 (46. 2) - - - 0. 012 0. 065 0. 035 2/3 DDT in the LAD artery (milliseconds)# Clinical Trial Results. org 11 (91. 7) 7 (53. 8) - 828+258 360+292 468 (261)-(676) <0. 001 0. 13 - - 750 (446)-(1054) 257 (-65)-(580) 0. 001 Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

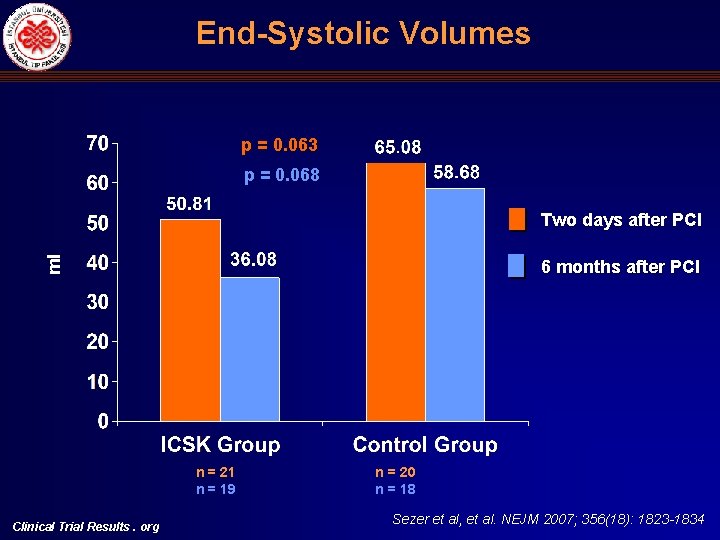
End Systolic Volumes p = 0. 063 p = 0. 068 ml Two days after PCI 6 months after PCI n = 21 n = 19 Clinical Trial Results. org n = 20 n = 18 Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

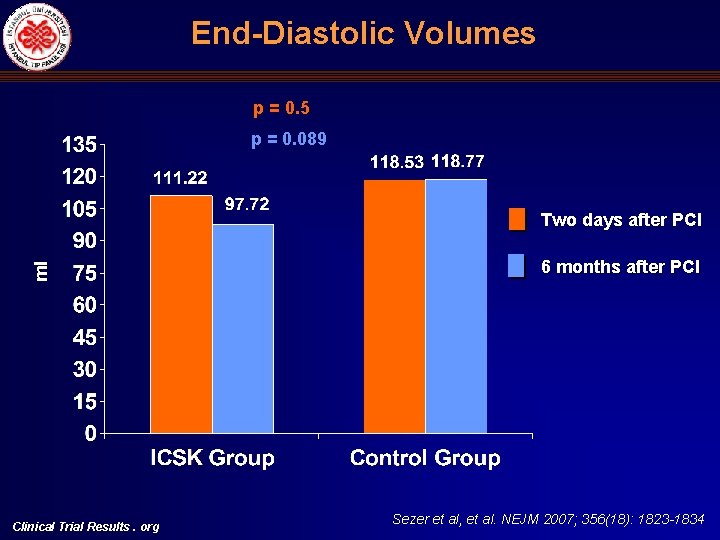
End Diastolic Volumes p = 0. 5 p = 0. 089 ml Two days after PCI Clinical Trial Results. org 6 months after PCI Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

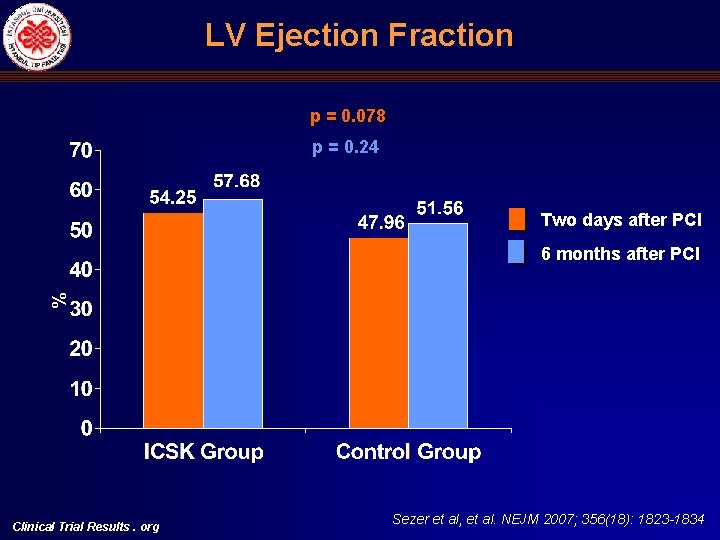
LV Ejection Fraction p = 0. 078 p = 0. 24 Two days after PCI % 6 months after PCI Clinical Trial Results. org Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

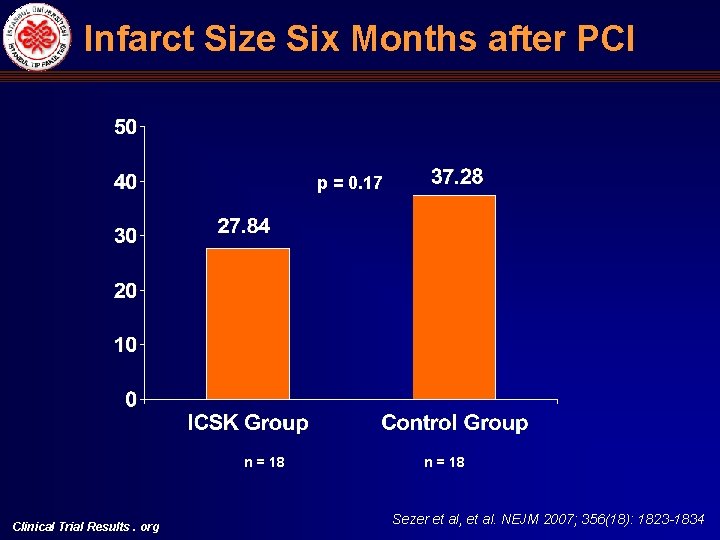
Infarct Size Six Months after PCI p = 0. 17 n = 18 Clinical Trial Results. org n = 18 Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

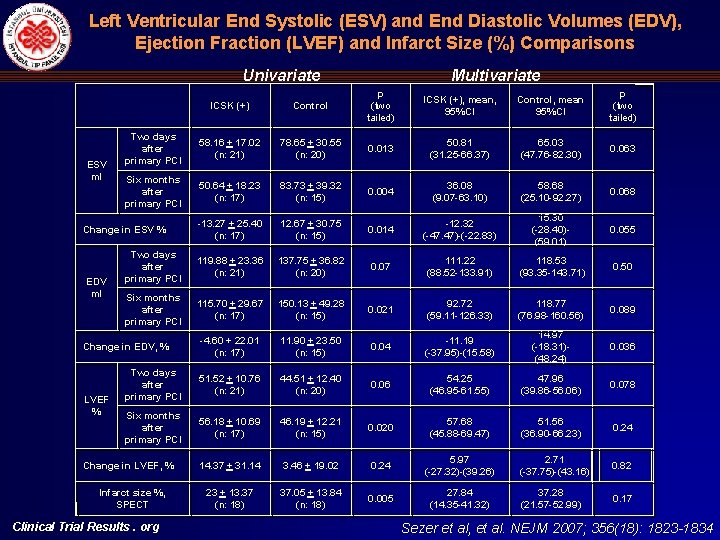
Left Ventricular End Systolic (ESV) and End Diastolic Volumes (EDV), Ejection Fraction (LVEF) and Infarct Size (%) Comparisons Univariate ESV ml ICSK (+) Control p (two tailed) ICSK (+), mean, 95%CI Control, mean 95%CI p (two tailed) Two days after primary PCI 58. 16 + 17. 02 (n: 21) 78. 65 + 30. 55 (n: 20) 0. 013 50. 81 (31. 25 -66. 37) 65. 03 (47. 76 -82. 30) 0. 063 Six months after primary PCI 50. 64 + 18. 23 (n: 17) 83. 73 + 39. 32 (n: 15) 0. 004 36. 08 (9. 07 -63. 10) 58. 68 (25. 10 -92. 27) 0. 068 -13. 27 + 25. 40 (n: 17) 12. 67 + 30. 75 (n: 15) 0. 014 -12. 32 (-47. 47)-(-22. 83) 15. 30 (-28. 40)(59. 01) 0. 055 Two days after primary PCI 119. 88 + 23. 36 (n: 21) 137. 75 + 36. 82 (n: 20) 0. 07 111. 22 (88. 52 -133. 91) 118. 53 (93. 35 -143. 71) 0. 50 Six months after primary PCI 115. 70 + 29. 67 (n: 17) 150. 13 + 49. 28 (n: 15) 0. 021 92. 72 (59. 11 -126. 33) 118. 77 (76. 98 -160. 56) 0. 089 -4. 60 + 22. 01 (n: 17) 11. 90 + 23. 50 (n: 15) 0. 04 -11. 19 (-37. 95)-(15. 58) 14. 97 (-18. 31)(48. 24) 0. 036 Two days after primary PCI 51. 52 + 10. 76 (n: 21) 44. 51 + 12. 40 (n: 20) 0. 06 54. 25 (46. 95 -61. 55) 47. 96 (39. 86 -56. 06) 0. 078 Six months after primary PCI 56. 18 + 10. 69 (n: 17) 46. 19 + 12. 21 (n: 15) 0. 020 57. 68 (45. 88 -69. 47) 51. 56 (36. 90 -66. 23) 0. 24 14. 37 + 31. 14 3. 46 + 19. 02 0. 24 5. 97 (-27. 32)-(39. 26) 2. 71 (-37. 75)-(43. 16) 0. 82 23 + 13. 37 (n: 18) 37. 05 + 13. 84 (n: 18) 0. 005 27. 84 (14. 35 -41. 32) 37. 28 (21. 57 -52. 99) 0. 17 Change in ESV % EDV ml Change in EDV, % LVEF % Multivariate Change in LVEF, % Infarct size %, SPECT Clinical Trial Results. org Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

Comments and Conclusions Early phase results: • In this pilot trial, low dose intracoronary streptokinase administration immediately following primary PCI was compared with standard primary PCI without use of intracoronary streptokinase • Almost all indices of microvascular perfusion concordantly pointed out that use of intracoronary streptokinase immediately after primary PCI yields better perfusion at the microvascular level Clinical Trial Results. org Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

Comments and Conclusions (Cont. ) Late term results: • At six months, there was no significant difference between the two study groups with regards to left ventricular size of function and infarct size, although there were some trends favoring the streptokinase group. • The trial was not originally planned to be large enough to detect differences in long-term outcome, and indeed enrollment was terminated early based on the midterm data on microvascular perfusion. • Since trends in favor of the intracoronary streptokinase group were detected, it is possible that the study was underpowered for these analyses. Clinical Trial Results. org Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834

Comments and Conclusions (Cont. ) • The finding of the current study supports the in situ formed (autochthonous) microvascular thrombus hypothesis and pointed out that this thrombus should be taken into consideration for achieving more efficient reperfusion at microvascular level during primary PCI. • The results of the study should be confirmed by a larger randomized study before applying this treatment modality in daily cardiology practice. Clinical Trial Results. org Sezer et al, et al. NEJM 2007; 356(18): 1823 -1834
 Streptokinase mechanism of action
Streptokinase mechanism of action Streptokinase mechanism of action
Streptokinase mechanism of action Time goal for fibrinolytic checklist
Time goal for fibrinolytic checklist John 14
John 14 After me after me after me
After me after me after me Yaygın bileşikler ve formülleri
Yaygın bileşikler ve formülleri Headed concrete anchors
Headed concrete anchors Ncvd pci registry
Ncvd pci registry Accelerated graphics port year created
Accelerated graphics port year created Tailor pci
Tailor pci Aspm windows
Aspm windows Pci-32765
Pci-32765 Sox pci compliance wikipedia
Sox pci compliance wikipedia Pci magistrala
Pci magistrala Proyecto curricular institucional
Proyecto curricular institucional Jill miller pci
Jill miller pci Niveaux pci dss
Niveaux pci dss Pci en salud
Pci en salud Mponline kiosk login
Mponline kiosk login Pxi-pci8330
Pxi-pci8330 Pci goal
Pci goal Pci tutorial
Pci tutorial Agp pci
Agp pci Ukrainese
Ukrainese Barramento pci
Barramento pci Modle
Modle Pci cetem
Pci cetem Pci en salud
Pci en salud Pci class code
Pci class code Pci en salud
Pci en salud Geometria angular exemplos
Geometria angular exemplos Umt confession
Umt confession Microsoft from back doors patch gov
Microsoft from back doors patch gov Pci
Pci Vanjska sabirnica
Vanjska sabirnica Pci reading program
Pci reading program