Honors Chemistry Chapter 5 Gases 5 1 Gases












- Slides: 25

Honors Chemistry Chapter 5 Gases

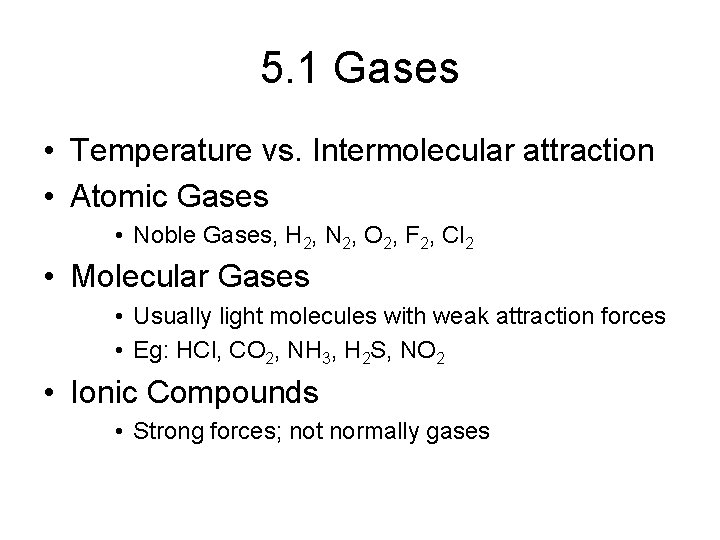
5. 1 Gases • Temperature vs. Intermolecular attraction • Atomic Gases • Noble Gases, H 2, N 2, O 2, F 2, Cl 2 • Molecular Gases • Usually light molecules with weak attraction forces • Eg: HCl, CO 2, NH 3, H 2 S, NO 2 • Ionic Compounds • Strong forces; not normally gases

5. 2 Pressure • • • Force per unit area P = F/A N/m 2 unit defined as Pascal (Pa) Standard air pressure = 101. 325 k. Pa Also called 1 atmosphere (atm) Measured by unequal mercury levels Manometers and barometers Common unit called mm. Hg (or Torr) Standard air pressure = 760 mm. Hg

5. 2 Dimensional Analysis • Convert 75. 0 k. Pa to mm. Hg • 75. 0 k. Pa 760 mm. Hg ------ x -------- = 563 mm. Hg 1 101. 325 k. Pa • Try this one • Convert 1. 25 atm to k. Pa

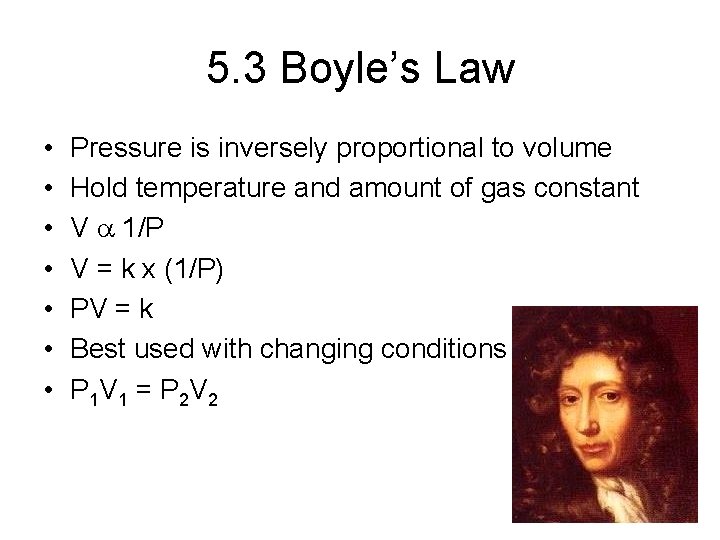
5. 3 Boyle’s Law • • Pressure is inversely proportional to volume Hold temperature and amount of gas constant V a 1/P V = k x (1/P) PV = k Best used with changing conditions P 1 V 1 = P 2 V 2

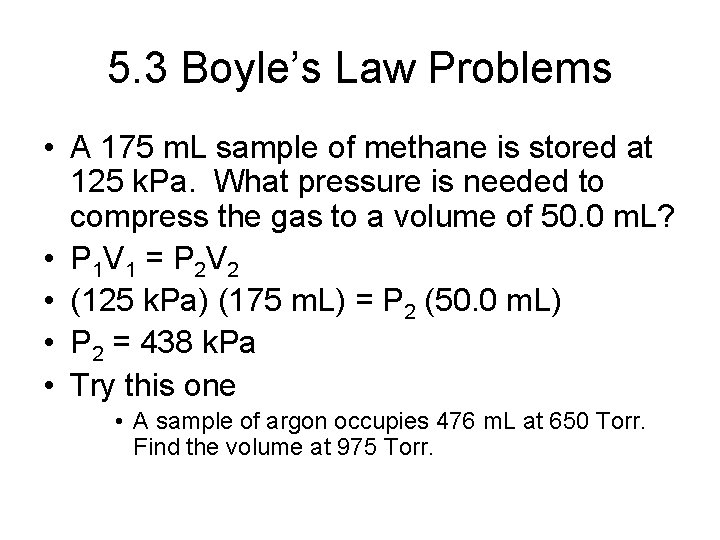
5. 3 Boyle’s Law Problems • A 175 m. L sample of methane is stored at 125 k. Pa. What pressure is needed to compress the gas to a volume of 50. 0 m. L? • P 1 V 1 = P 2 V 2 • (125 k. Pa) (175 m. L) = P 2 (50. 0 m. L) • P 2 = 438 k. Pa • Try this one • A sample of argon occupies 476 m. L at 650 Torr. Find the volume at 975 Torr.

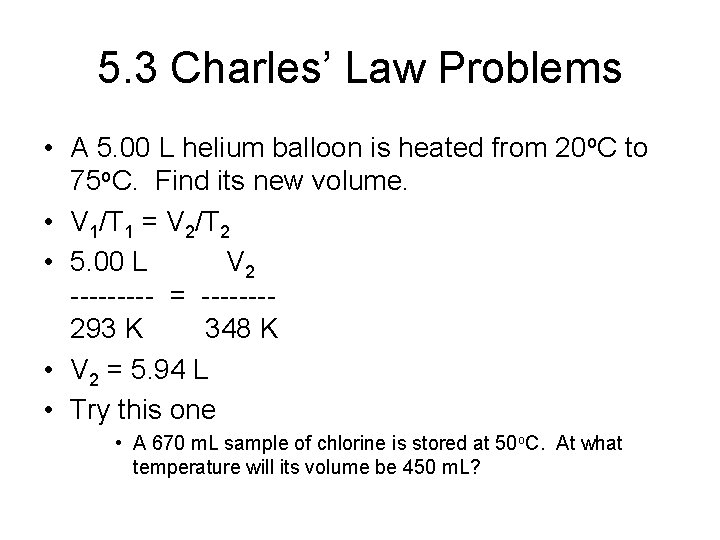
5. 3 Charles’ Law • • Also credited to Gay-Lussac Volume is directly proportional to temperature Hold pressure and amount of gas constant Va. T V = k. T Linear relationship Must use Kelvins! V 1 V 2 --- = --T 1 T 2

5. 3 Charles’ Law Problems • A 5. 00 L helium balloon is heated from 20 o. C to 75 o. C. Find its new volume. • V 1/T 1 = V 2/T 2 • 5. 00 L V 2 ----- = -------293 K 348 K • V 2 = 5. 94 L • Try this one • A 670 m. L sample of chlorine is stored at 50 o. C. At what temperature will its volume be 450 m. L?

5. 3 More Gas Laws • Another form of Charles’ Law • Pressure is directly proportional to temperature • P = k. T • P 1/T 1 = P 2/T 2 • Avogadro’s Law • Volume is directly proportional to the amount of gas present • Van • Volume relationships in chemical reactions

5. 3 Avogadro’s Law Problems • How many liters of hydrogen are needed to completely react with 1 liter of oxygen? • 2 H 2 + O 2 2 H 2 O • 2 mol hydrogen react with 1 mol oxygen • V a n, so…. • 2 L hydrogen react with 1 L oxygen • Try this one • How many liters of ammonia are formed when 1 L of hydrogen reacts with excess nitrogen?

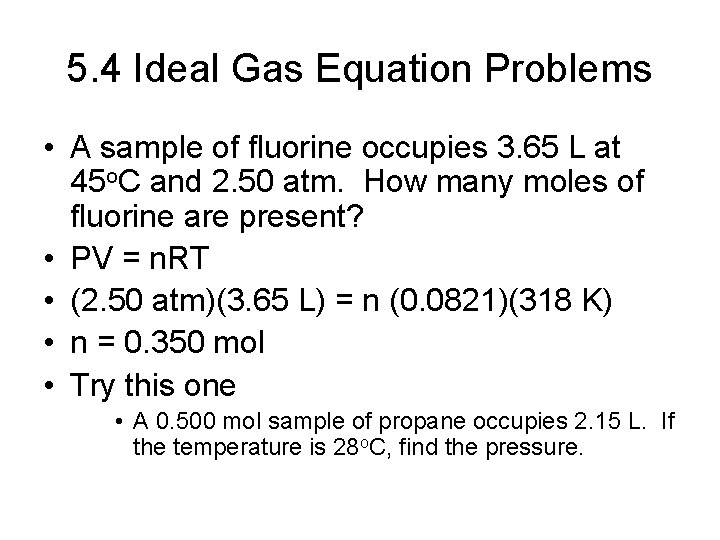
5. 4 The Ideal Gas Equation • Ideal Gas • No intermolecular attraction forces • Particles have no volume • Combine Boyle’s, Charles, and Avogadro’s Laws • PV = n. RT • STP = 1 atm, 273 K • Molar volume of a gas = 22. 414 L at STP • R = 0. 0821 atm L / mol K

5. 4 Ideal Gas Equation Problems • A sample of fluorine occupies 3. 65 L at 45 o. C and 2. 50 atm. How many moles of fluorine are present? • PV = n. RT • (2. 50 atm)(3. 65 L) = n (0. 0821)(318 K) • n = 0. 350 mol • Try this one • A 0. 500 mol sample of propane occupies 2. 15 L. If the temperature is 28 o. C, find the pressure.

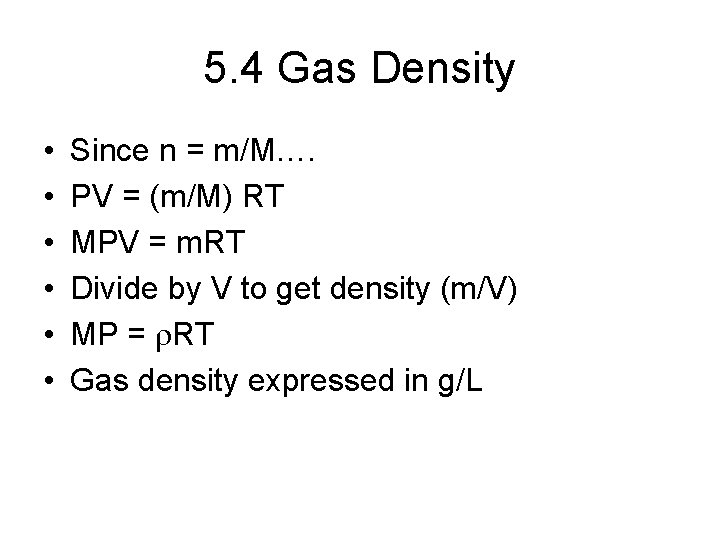
5. 4 Gas Density • • • Since n = m/M…. PV = (m/M) RT MPV = m. RT Divide by V to get density (m/V) MP = r. RT Gas density expressed in g/L

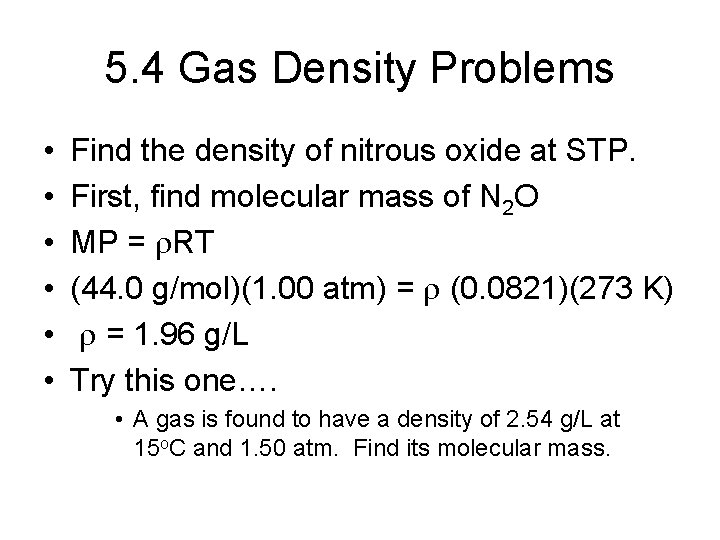
5. 4 Gas Density Problems • • • Find the density of nitrous oxide at STP. First, find molecular mass of N 2 O MP = r. RT (44. 0 g/mol)(1. 00 atm) = r (0. 0821)(273 K) r = 1. 96 g/L Try this one…. • A gas is found to have a density of 2. 54 g/L at 15 o. C and 1. 50 atm. Find its molecular mass.

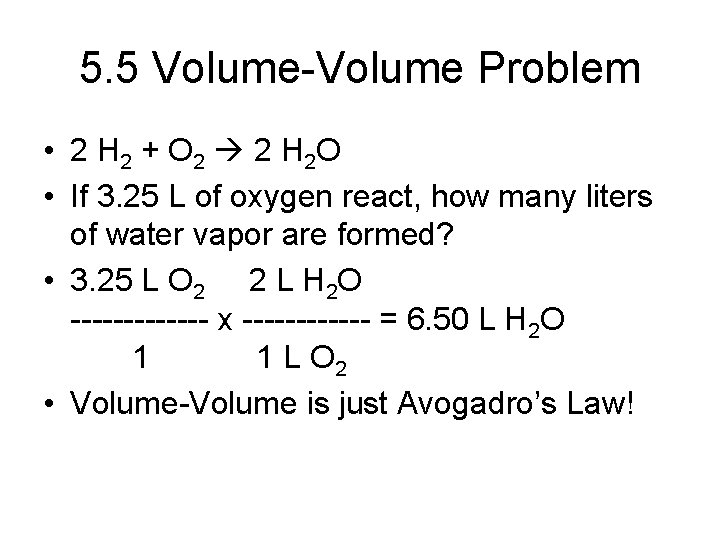
5. 5 Gas Stoichiometry • • Mass-Mass problems (review) Volume-Volume problems Volume is proportional to moles, so…. Mol relationship from reaction can be used directly • No conversions needed!

5. 5 Volume-Volume Problem • 2 H 2 + O 2 2 H 2 O • If 3. 25 L of oxygen react, how many liters of water vapor are formed? • 3. 25 L O 2 2 L H 2 O ------- x ------ = 6. 50 L H 2 O 1 1 L O 2 • Volume-Volume is just Avogadro’s Law!

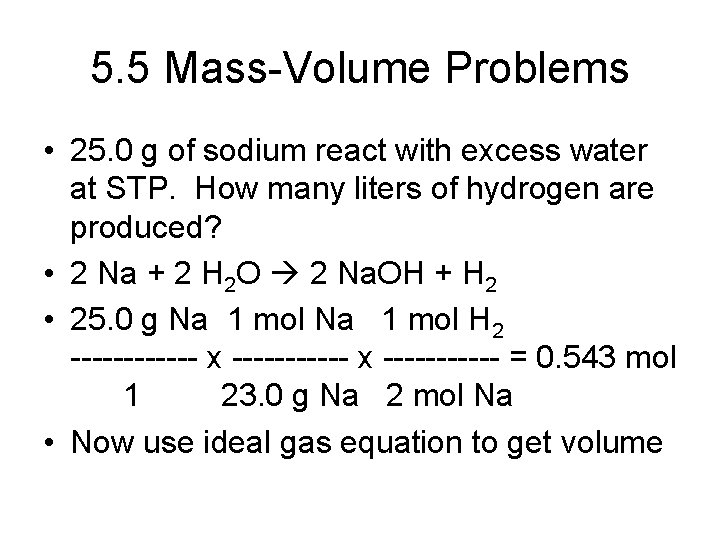
5. 5 Mass-Volume Problems • • Key step – get to moles! Mass conversion – use molecular mass Volume conversion – use gas equation Need to know temperature and pressure conditions

5. 5 Mass-Volume Problems • 25. 0 g of sodium react with excess water at STP. How many liters of hydrogen are produced? • 2 Na + 2 H 2 O 2 Na. OH + H 2 • 25. 0 g Na 1 mol H 2 ------ x ----------- = 0. 543 mol 1 23. 0 g Na 2 mol Na • Now use ideal gas equation to get volume

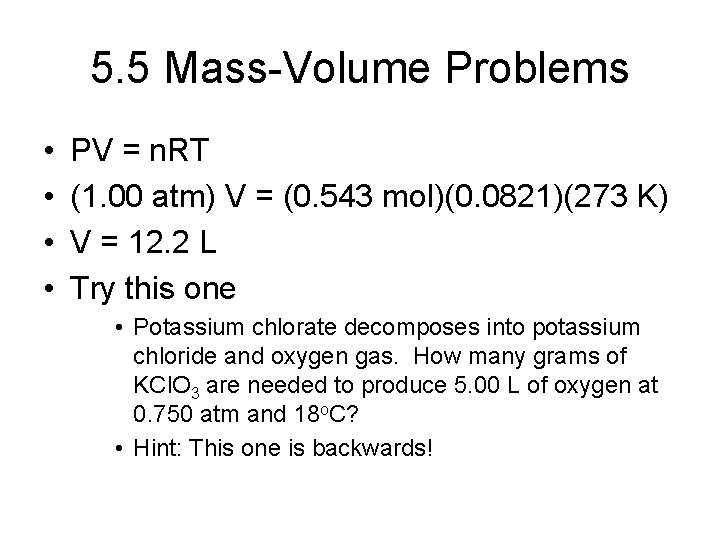
5. 5 Mass-Volume Problems • • PV = n. RT (1. 00 atm) V = (0. 543 mol)(0. 0821)(273 K) V = 12. 2 L Try this one • Potassium chlorate decomposes into potassium chloride and oxygen gas. How many grams of KCl. O 3 are needed to produce 5. 00 L of oxygen at 0. 750 atm and 18 o. C? • Hint: This one is backwards!

5. 6 Dalton’s Law • Partial pressure – the pressure of an individual gas in a mixture of gases • Total pressure of a mixture equals the sum of the partial pressures of each gas • Pt = P 1 + P 2 + P 3 +. . . • Partial pressure is proportional to the mol fraction (X 1 = n 1 / nt) • P 1 = X 1 P t

5. 6 Dalton’s Law • 2. 00 mol He is mixed with 1. 00 mol Ar. Find the partial pressure of each at 1. 75 atm pressure. • XHe = 2. 00 mol / 3. 00 mol = 0. 667 • XAr = 1. 00 mol / 3. 00 mol = 0. 333 • PHe = (0. 667) (1. 75 atm) = 1. 17 atm • PAr = (0. 333) (1. 75 atm) = 0. 583 atm • Try this. . . • Find the partial pressure of oxygen in air if it makes up 21% of the Earth’s atmosphere by volume. (Note: The volume gives you the mole ratio because of Avogadro’s law. )

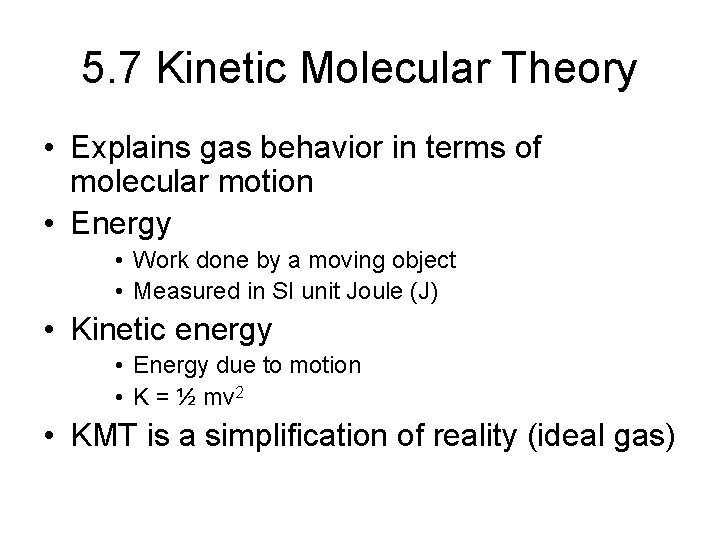
5. 7 Kinetic Molecular Theory • Explains gas behavior in terms of molecular motion • Energy • Work done by a moving object • Measured in SI unit Joule (J) • Kinetic energy • Energy due to motion • K = ½ mv 2 • KMT is a simplification of reality (ideal gas)

5. 7 Kinetic Molecular Theory • Gas molecules are separated by great distances • They can be treated as “point masses” • Gas molecules are in constant random motion • Frequent elastic collisions (no energy lost) • No attractive or repulsive forces • Average K is proportional to Temperature

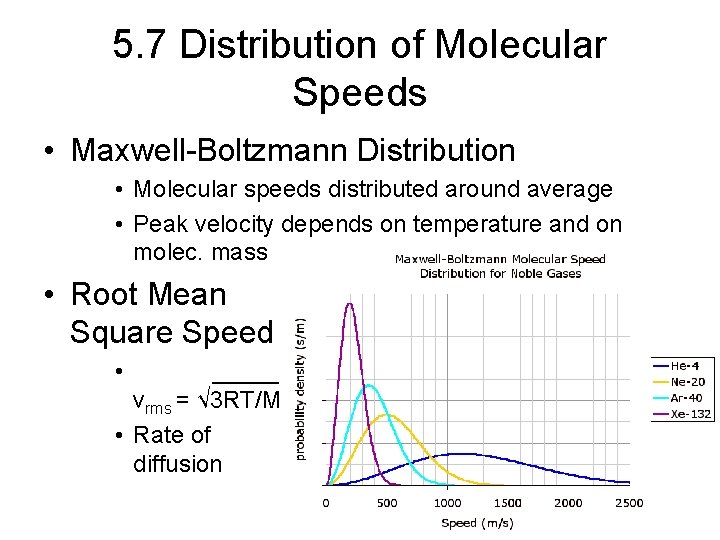
5. 7 Distribution of Molecular Speeds • Maxwell-Boltzmann Distribution • Molecular speeds distributed around average • Peak velocity depends on temperature and on molec. mass • Root Mean Square Speed • _____ vrms = √ 3 RT/M • Rate of diffusion

5. 8 Deviations from Ideal Behavior • We made approximations! • Point masses • No intermolecular forces • These approximations become bad at. . . • High pressure • Low temperature • Liquefaction • van der Waals Equation • (P + an 2/V 2) (V – nb) = n. RT
 Kuei honors chemistry
Kuei honors chemistry Chemistry unit 4 review answer key
Chemistry unit 4 review answer key Honors chemistry summer assignment
Honors chemistry summer assignment Bond order
Bond order Chapter 11 review gases section 1
Chapter 11 review gases section 1 Chapter 3 geometry test
Chapter 3 geometry test Precalculus honors chapter 1 test
Precalculus honors chapter 1 test Creighton honors program
Creighton honors program James scholar honors program
James scholar honors program Policy guidelines on awards and recognition
Policy guidelines on awards and recognition Ucsb honors college
Ucsb honors college Honors physics semester 2 review
Honors physics semester 2 review Honors biology ecology test
Honors biology ecology test Uncle sam's toolbox assignment answers
Uncle sam's toolbox assignment answers Quadrilateral test review
Quadrilateral test review Honors earth science
Honors earth science Honors math 3
Honors math 3 Hilton honors military program
Hilton honors military program Honors project
Honors project Honors biology properties of water lab
Honors biology properties of water lab Honors geometry parallel lines and transversals worksheet
Honors geometry parallel lines and transversals worksheet Tulane honors program
Tulane honors program Honors physics projectile motion test
Honors physics projectile motion test Honors biology unit 4 test
Honors biology unit 4 test Smc scholars classes
Smc scholars classes Golden opulence sundae
Golden opulence sundae