Gypsum Sulfate Minerals More than 100 different minerals












- Slides: 23

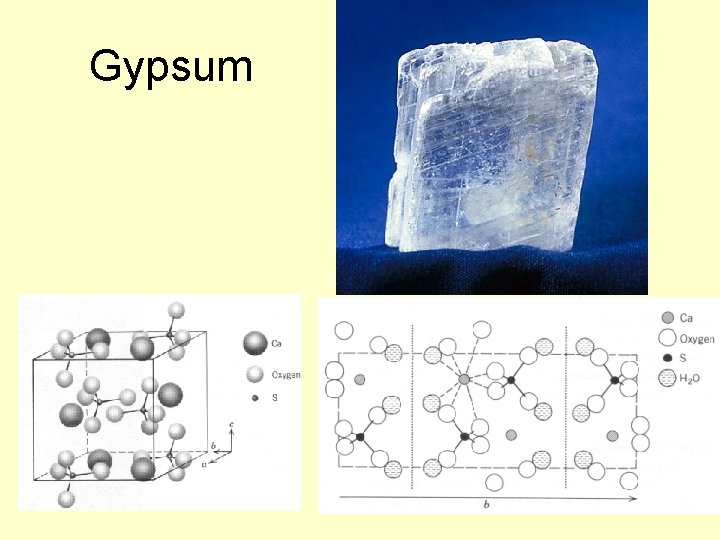
Gypsum

Sulfate Minerals • More than 100 different minerals, separated into hydrous (with H 2 O) or anhydrous (without H 2 O) groups • Gypsum (Ca. SO 4*2 H 2 O) and anhydrite (Ca. SO 4) are the most common of the sulfate minerals • Gypsum typically forms in evaporitic basins – a polymorph of anhydrite (g-Ca. SO 4) forms when the gypsum is later dehydrated)

Halide Minerals • Minerals contianing halogen elements as dominant anion (Cl- or F- typically) • Halite (Na. Cl) and Sylvite (KCl) form in VERY concentrated evaporitic waters – they are extremely soluble in water, indicate more complete evaporation than does gypsum • Fluorite (Ca. F 2) more typically occurs in veins associated with hydrothermal waters (F- in hydrothermal solutions is typically much higher – leached out of parent minerals such as biotites, pyroxenes, hornblendes or apatite)

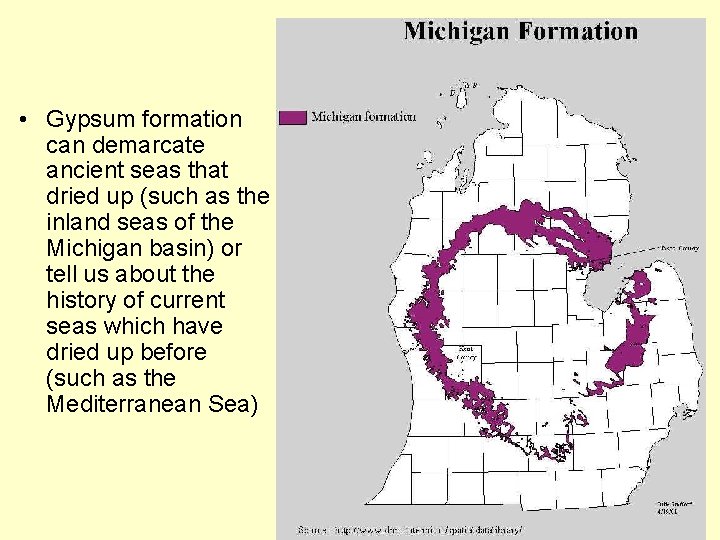
• Gypsum formation can demarcate ancient seas that dried up (such as the inland seas of the Michigan basin) or tell us about the history of current seas which have dried up before (such as the Mediterranean Sea)

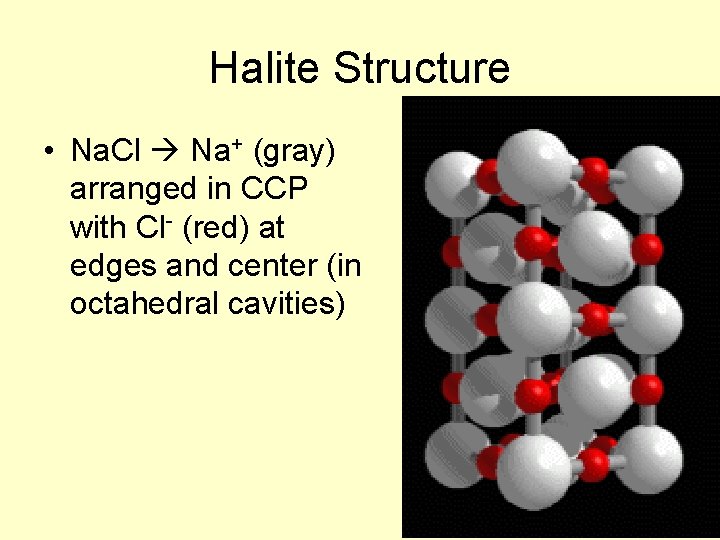
Halite Structure • Na. Cl Na+ (gray) arranged in CCP with Cl- (red) at edges and center (in octahedral cavities)

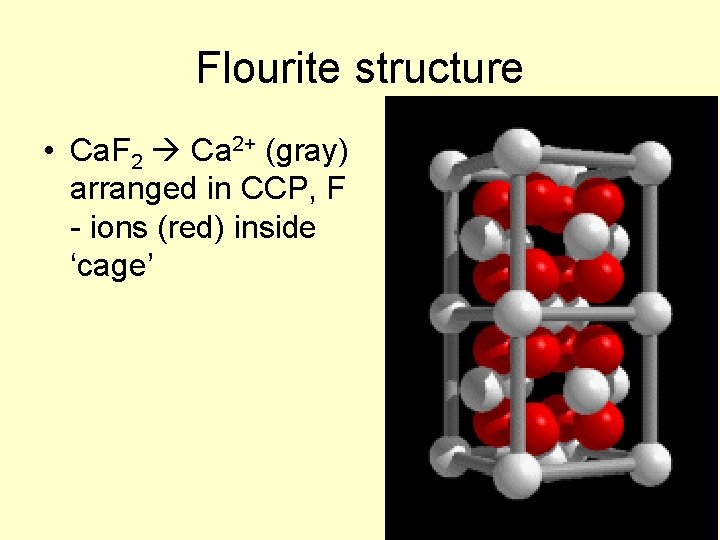
Flourite structure • Ca. F 2 Ca 2+ (gray) arranged in CCP, F - ions (red) inside ‘cage’

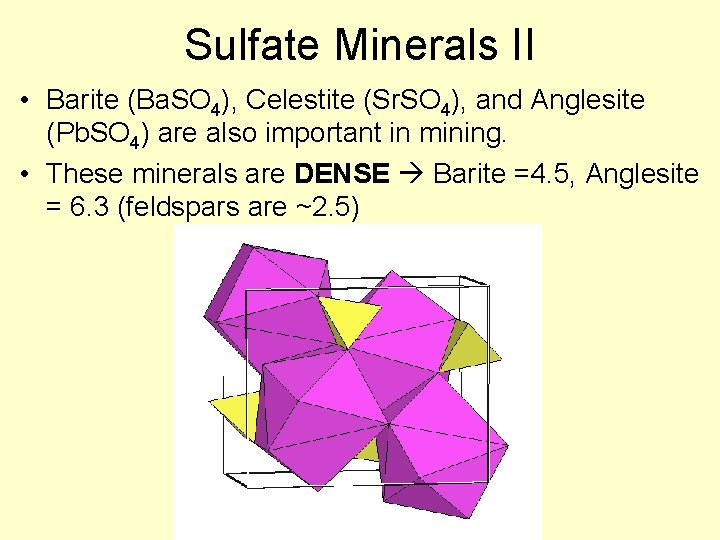
Sulfate Minerals II • Barite (Ba. SO 4), Celestite (Sr. SO 4), and Anglesite (Pb. SO 4) are also important in mining. • These minerals are DENSE Barite =4. 5, Anglesite = 6. 3 (feldspars are ~2. 5)

Barite, Celestite, Anglesite • Metals bond with sulfate much more easily, and thus are generally more insoluble – they do not require formation in evaporitic basins • What do they indicate then? Ba, Pb, Sr – very low SO 4 2 - Lots of SO 42 Not very much Ba, Sr, Pb

Just silica… • Chert – extremely fine grained quartz – Forms as nodules in limestone, recrystallization of siliceous fossils – Jasper – variety with hematite inclusions red – Flint – variety containing organic matter darker color • Chalcedony – microcrystaliine silica (very similar to low quartz, but different – it is yet uncertain how different…) typically shows banding, often colored to form an agate (rock formed of multiple bands of colored chalcedony) • Jasper – variety colored with inclusion of microcrystsalline oxides (often iron oxides = red) • Opal – a hydrogel (a solid solution of water in silica) – forms initially as water + silica colloids, then slowly the water diffuses into the silica making it amorphous (no XRD pattern!) – Some evidence opal slowly recrystallizes to chalcedony

Opal - Gemstone

Agates

Oxides - Oxyhydroxides • Fe. OOH minerals Goethite or Limonite (Fe. OOH) important alteration products of weathering Fe-bearing minerals • Hematite (Fe 2 O 3) primary iron oxide in Banded Iron Formations • Boehmite (Al. OOH) primary mineral in bauxite ores (principle Al ore) which forms in tropical soils • Mn oxides form Mn nodules in the oceans (estimated they cover 10 -30% of the deep Pacific floor) • Many other oxides important in metamorphic rocks…


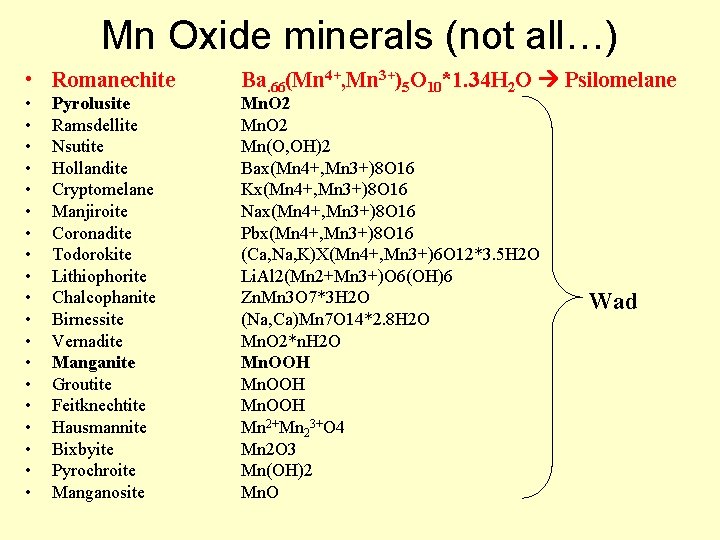
Mn oxides - oxyhydroxides • Mn exists as 2+, 3+, and 4+; oxide minerals are varied, complex, and hard to ID – ‘Wad’ soft (i. e. blackens your fingers), brown-black fine-grained Mn oxides – ‘Psilomelane’ hard (does not blacked fingers) grayblack botroyoidal, massive Mn oxides • XRD analyses do not easily distinguish different minerals, must combine with TEM, SEM, IR spectroscopy, and microprobe work

Mn Oxide minerals (not all…) • Romanechite • • • • • Pyrolusite Ramsdellite Nsutite Hollandite Cryptomelane Manjiroite Coronadite Todorokite Lithiophorite Chalcophanite Birnessite Vernadite Manganite Groutite Feitknechtite Hausmannite Bixbyite Pyrochroite Manganosite Ba. 66(Mn 4+, Mn 3+)5 O 10*1. 34 H 2 O Psilomelane Mn. O 2 Mn(O, OH)2 Bax(Mn 4+, Mn 3+)8 O 16 Kx(Mn 4+, Mn 3+)8 O 16 Nax(Mn 4+, Mn 3+)8 O 16 Pbx(Mn 4+, Mn 3+)8 O 16 (Ca, Na, K)X(Mn 4+, Mn 3+)6 O 12*3. 5 H 2 O Li. Al 2(Mn 2+Mn 3+)O 6(OH)6 Zn. Mn 3 O 7*3 H 2 O (Na, Ca)Mn 7 O 14*2. 8 H 2 O Mn. O 2*n. H 2 O Mn. OOH Mn 2+Mn 23+O 4 Mn 2 O 3 Mn(OH)2 Mn. O Wad

Al oxides • Aluminum occus in economic deposits principally as bauxite • Bauxite is a mixture of Al oxides and oxyhydroxides: – Diaspore - Al. O(OH) – Gibbsite - Al(OH)3 – Böhmite - Al. O(OH) • Al is a residual phase and bauxite occurs where weathering is extreme and thick layers of aluminum oxyhydroxide are left over

Iron Oxides • Interaction of dissolved iron with oxygen yields iron oxide and iron oxyhyroxide minerals • 1 st thing precipitated amorphous or extremely fine grained (nanocrystaliine) iron oxides called ferrihydrite Fe 2+ O 2

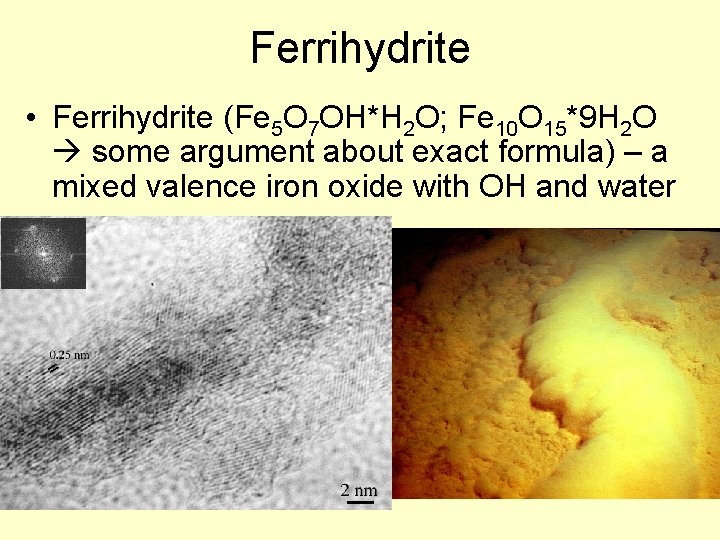
Ferrihydrite • Ferrihydrite (Fe 5 O 7 OH*H 2 O; Fe 10 O 15*9 H 2 O some argument about exact formula) – a mixed valence iron oxide with OH and water

Goethite • Ferrihydrite recrystallizes into Goethite (a. Fe. OOH) • There are other polymorphs of iron oxyhydroxides: – Lepidocrocite g-Fe. OOH – Akaganeite b-Fe. OOH

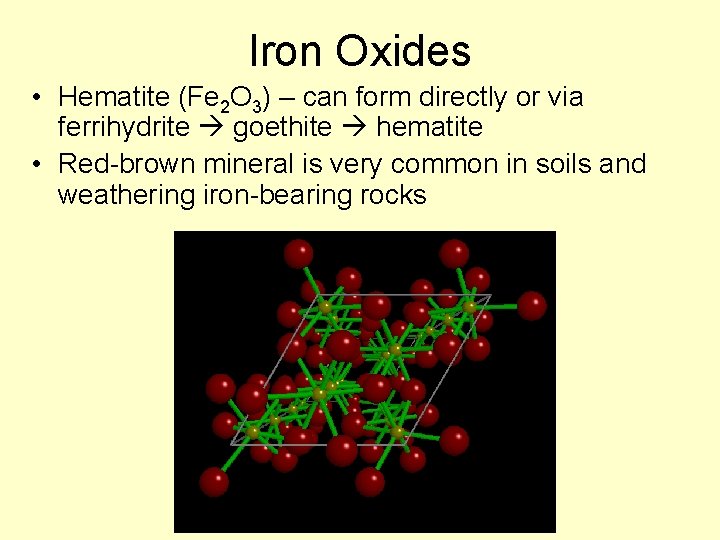
Iron Oxides • Hematite (Fe 2 O 3) – can form directly or via ferrihydrite goethite hematite • Red-brown mineral is very common in soils and weathering iron-bearing rocks

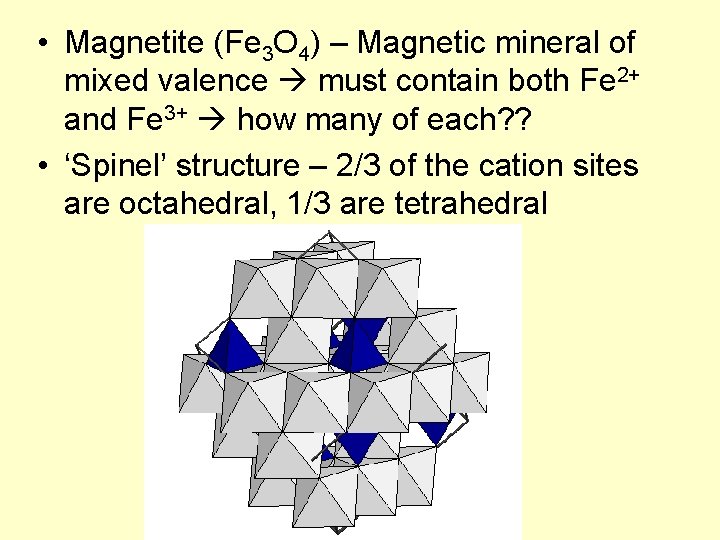
• Magnetite (Fe 3 O 4) – Magnetic mineral of mixed valence must contain both Fe 2+ and Fe 3+ how many of each? ? • ‘Spinel’ structure – 2/3 of the cation sites are octahedral, 1/3 are tetrahedral

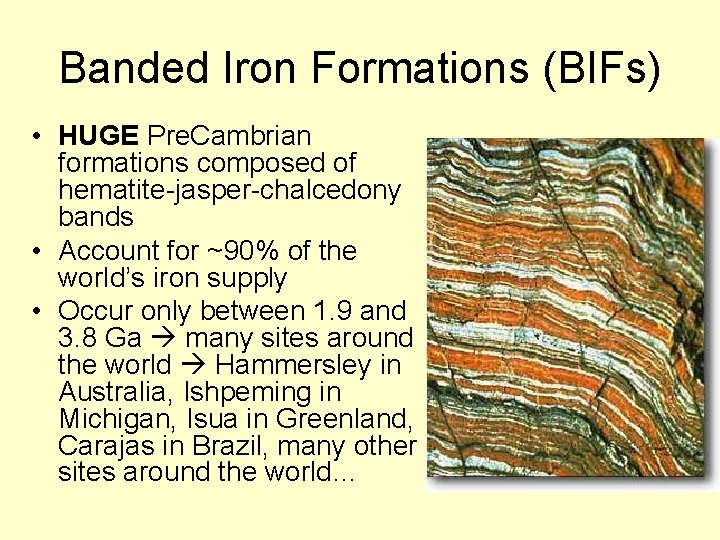
Banded Iron Formations (BIFs) • HUGE Pre. Cambrian formations composed of hematite-jasper-chalcedony bands • Account for ~90% of the world’s iron supply • Occur only between 1. 9 and 3. 8 Ga many sites around the world Hammersley in Australia, Ishpeming in Michigan, Isua in Greenland, Carajas in Brazil, many other sites around the world…

BIFs and bacteria • Early earth did not have free O 2, as microbial activity became widespread and photosynthetic organisms started generating O 2, the reduced species previously stable (without the O 2) oxidized – for Fe this results in formation of iron oxide minerals
 More more more i want more more more more we praise you
More more more i want more more more more we praise you More more more i want more more more more we praise you
More more more i want more more more more we praise you 100 100 100 100 100
100 100 100 100 100 Percent greater than 100 and less than 1
Percent greater than 100 and less than 1 Generous kelimesinin comparative hali
Generous kelimesinin comparative hali Half life more than 2 less than 4
Half life more than 2 less than 4 Better than god
Better than god Ammonium sulfate precipitation
Ammonium sulfate precipitation Normal cervical length
Normal cervical length Sulfate anion test
Sulfate anion test Which is an example of a polyatomic ion?h2co3-mg+ne+ -
Which is an example of a polyatomic ion?h2co3-mg+ne+ - Severe preeclampsia criteria
Severe preeclampsia criteria Magnesium copper oxide
Magnesium copper oxide Mgso4 side effects
Mgso4 side effects Reaction between sodium and oxygen
Reaction between sodium and oxygen Aluminum sulfate + calcium hydroxide
Aluminum sulfate + calcium hydroxide Sulphuric acid + aluminium
Sulphuric acid + aluminium Berotec nebs
Berotec nebs Cristal de sulfate de cuivre
Cristal de sulfate de cuivre Dic medical abbreviation
Dic medical abbreviation Copper sulfate and potassium iodide precipitate
Copper sulfate and potassium iodide precipitate Sulfate reducing bacteria
Sulfate reducing bacteria Baruim sulfate
Baruim sulfate Acute fulminating preeclampsia
Acute fulminating preeclampsia