GYPSUM GYPSUM In nature Gypsum Rock Pure gypsum










- Slides: 14

GYPSUM

GYPSUM Ø In nature : Gypsum Rock Ø Pure gypsum rock : Ca. SO 4. 2 H 2 O Ø Impurities : Mg. O, Al 2 O 3, Fe 2 O 3, Si. O 2, Ca. CO 3, Mg. CO 3. . .

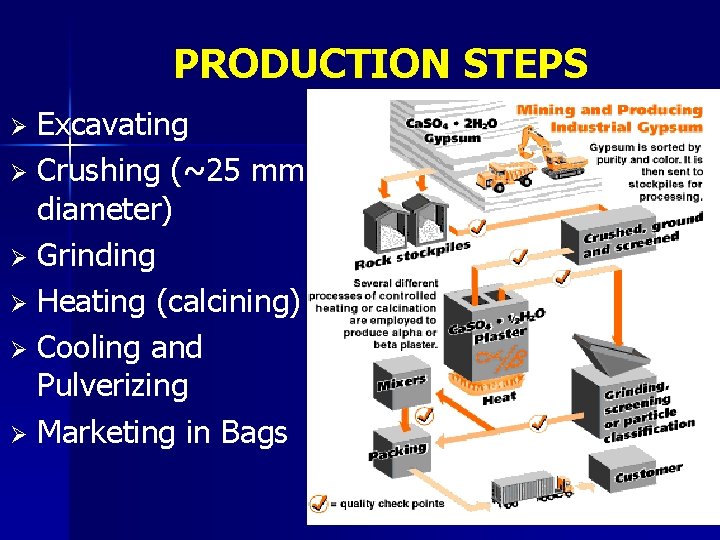
PRODUCTION STEPS Excavating Ø Crushing (~25 mm diameter) Ø Grinding Ø Heating (calcining) Ø Cooling and Pulverizing Ø Marketing in Bags Ø

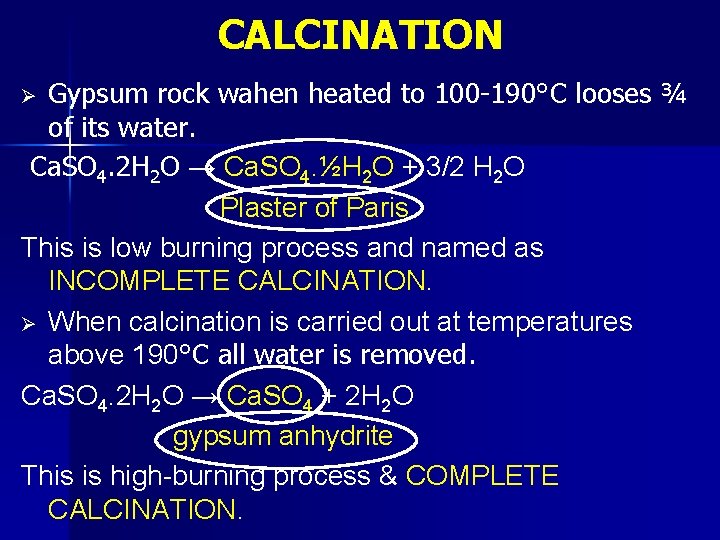
CALCINATION Gypsum rock wahen heated to 100 -190°C looses ¾ of its water. Ca. SO 4. 2 H 2 O → Ca. SO 4. ½H 2 O + 3/2 H 2 O Plaster of Paris This is low burning process and named as INCOMPLETE CALCINATION. Ø When calcination is carried out at temperatures above 190°C all water is removed. Ca. SO 4. 2 H 2 O → Ca. SO 4 + 2 H 2 O gypsum anhydrite This is high-burning process & COMPLETE CALCINATION. Ø

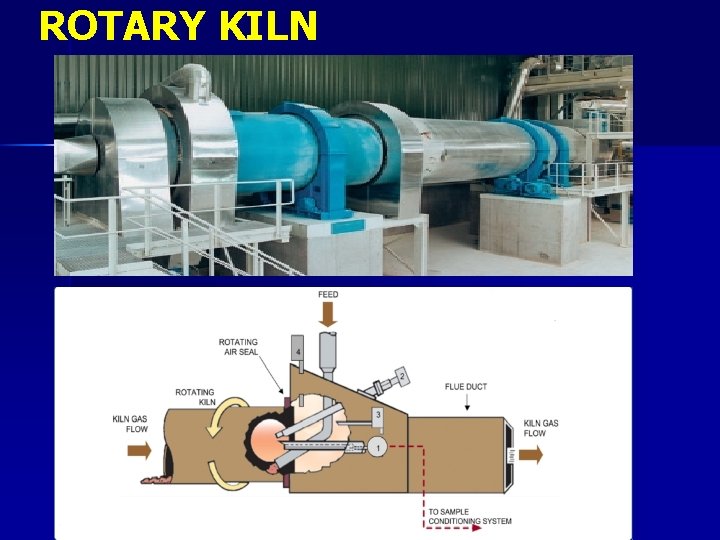
Ø Both of these products form gypsum rock by recombining with water. Ca. SO 4. ½H 2 O + 3/2 H 2 O → Ca. SO 4. 2 H 2 O Ca. SO 4 + 2 H 2 O → Ca. SO 4. 2 H 2 O Calcination process is carried out in two types of kilns. n Kettle Kilns n Rotary Kilns Ø

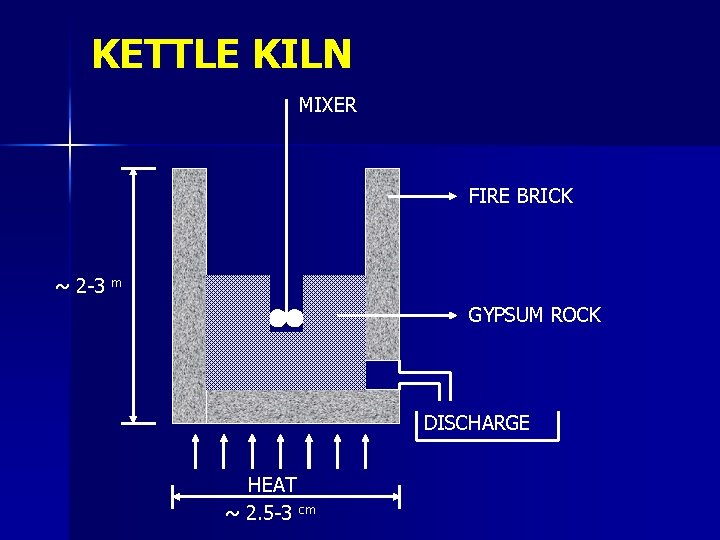
KETTLE KILN MIXER FIRE BRICK ~ 2 -3 m GYPSUM ROCK DISCHARGE HEAT ~ 2. 5 -3 cm

ROTARY KILN

GYPSUM PLASTERS Ø Obtained by Incomplete Calcination (Ca. SO 4. ½H 2 O) 1. Plaster of Paris : is formed by incomplete calcination at 100 -190°C. No admixtures are found. Hard Wall Plaster of Paris + Admixtures (Glue, Sand. . . ) 2.

Ø Obtained by Complete Calcination (Ca. SO 4) 1. Flooring Plaster (Ca. SO 4 with no impurities) 2. Hard Finish Plaster (Ca. SO 4 + Al 2(SO 4)3) (Ca. SO 4 + Na 2 B 4 O 7)

PROPERTIES & USES OF GYPSUM PLASTERS Ø Plaster of Paris – Setting time ~5 -20 min. – Used for sculpturing, ornamental work, small repair work

PROPERTIES & USES OF GYPSUM PLASTERS Ø Hard Wall Plaster – Setting time ~1 hr – Compressive strength ~7 MPa – Admixtures result in increased plasticity & setting time & reduced shrinkage – Can be used for plastering walls – Production of prefabricated structural units – Masonry bricks & blocks

PROPERTIES & USES OF GYPSUM PLASTERS Ø Flooring, Hard Finish Plaster – Setting time ~1 -16 hrs – Compressive strength > 7 MPa – Can be used for producing prefabricated units, masonry bricks & blocks & flooring & pavement bricks & tiles.

PROPERTIES & USES OF GYPSUM PLASTERS Gypsum often serves as a fire proofing material even though its strength is destroyed by long continuous heat. It forms a powder covering the surface which acts as an effective insulator. Ø Gypsum products tend to disintegrate when exposed to moisture. Therefore, they should not be used for exterior work & for moist interiors. (NON-HYDRAULIC) Ø

BY-PRODUCT GYPSUM Phospogypsum – Major by-product of phosporic acid production n Desulfogypsum – Obtained from the desulfurization of combustion gases in coal burning power plants (Harmful SO 2 gas is turned into Ca. SO 4. 2 H 2 O n
 Igneous rock to metamorphic rock
Igneous rock to metamorphic rock Pumice extrusive or intrusive
Pumice extrusive or intrusive Chapter 3 standardized test practice answers
Chapter 3 standardized test practice answers Rock cycle
Rock cycle A rock climber's shoe loosens a rock and her climbing buddy
A rock climber's shoe loosens a rock and her climbing buddy Compaction and cementation
Compaction and cementation Diagram of rock cycle
Diagram of rock cycle Adventure sports bungee jumping
Adventure sports bungee jumping Nature and nature's law lay hid in night meaning
Nature and nature's law lay hid in night meaning Determinace lidské psychiky
Determinace lidské psychiky Gypsum board uses
Gypsum board uses Lost wax pattern technique
Lost wax pattern technique Disposal of gypsum waste
Disposal of gypsum waste Gypsum board non-contact measuring system
Gypsum board non-contact measuring system Type of gypsum
Type of gypsum