General Pathology II Cell Death Jaroslava Dukov Inst


























- Slides: 35

General Pathology - II Cell Death Jaroslava Dušková Inst. Pathol. , 1 st Med. Faculty, Charles Univ. Prague http: //www 1. lf 1. cuni. cz/~jdusk/

Death irreversible damage of the morphological & functional integrity of cells organism

Cell Death apoptosis v necrosis v

Apoptosis induced (from outside) or v genetically programmed cell v death (cell execution / suicide) logical and functional contrary to mitosis v a system for the removal of unnecessary, aged, or damaged cells v

Necrosis cell death caused from external insult

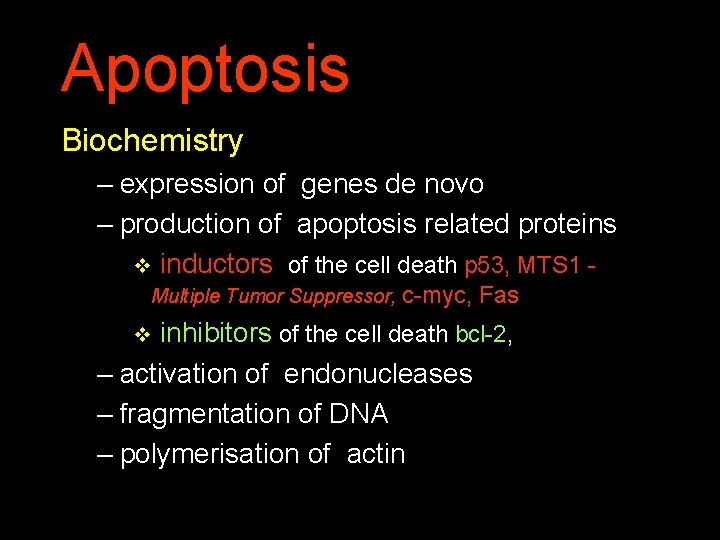
Apoptosis Biochemistry – expression of genes de novo – production of apoptosis related proteins v inductors of the cell death p 53, MTS 1 Multiple Tumor Suppressor, c-myc, Fas inhibitors of the cell death bcl-2, – activation of endonucleases – fragmentation of DNA – polymerisation of actin v

Apoptosis -1 • Triggered by a wide range of stimuli. • Cell surface receptors like Fas or tumor necrosis factor receptor 1 (TNFR 1). • Interplay of proapoptotic (Bax, Bad, Bik, and Bim) and antiapoptotic (Bcl-2 and Bcl-XL) proteins

http: //www. sigmaaldrich. com

Oxidative Damage -1 • Endogenous production of reactive oxygen by mitochondria. • Increased permeability transition (PT) in the mitochondrial membrane

Oxidative damage -2 u u u Apoptogenic factors leak into the cytoplasm cytochrome c and apo - inducing factor (AIF) a cascade of proteolytic activity DNA v fragmentation v mutations v cell death.

Mitochondria in Apoptosis Oxidative damage - 3 u Bcl-2 and Bcl-X – prevent pore formation – block the release of cytochrome c from the mitochondria – prevent activation of the caspase cascade and apoptosis.

Caspase Cascade -1 u Caspases - a class of cysteine proteases involved in apoptosis. u A proteolytic cascade activates enzymes that subsequently degrade cellular targets.

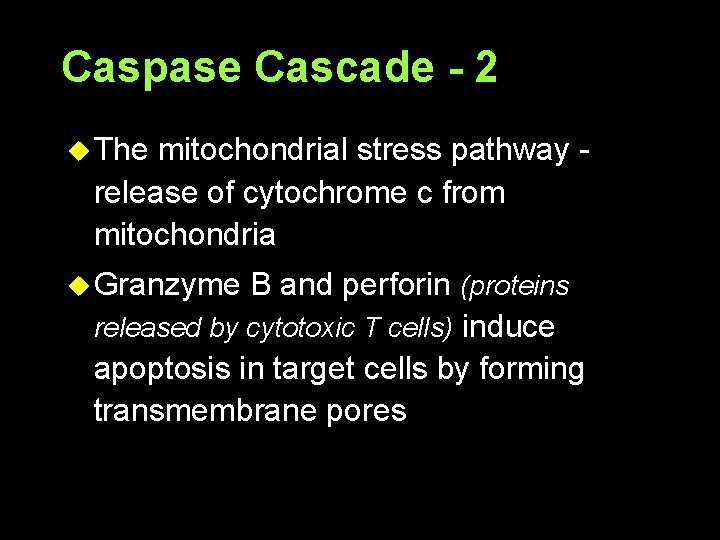
Caspase Cascade - 2 u The mitochondrial stress pathway release of cytochrome c from mitochondria u Granzyme B and perforin (proteins released by cytotoxic T cells) induce apoptosis in target cells by forming transmembrane pores

Caspase Cascade - 3 u Caspase-activated DNAse (CAD) may be activated through the cleavage of its associated inhibitor ICAD. CAD is then able to interact with components such as topoisomerase II (Topo II) to condense chromatin and lead to DNA fragmentation.

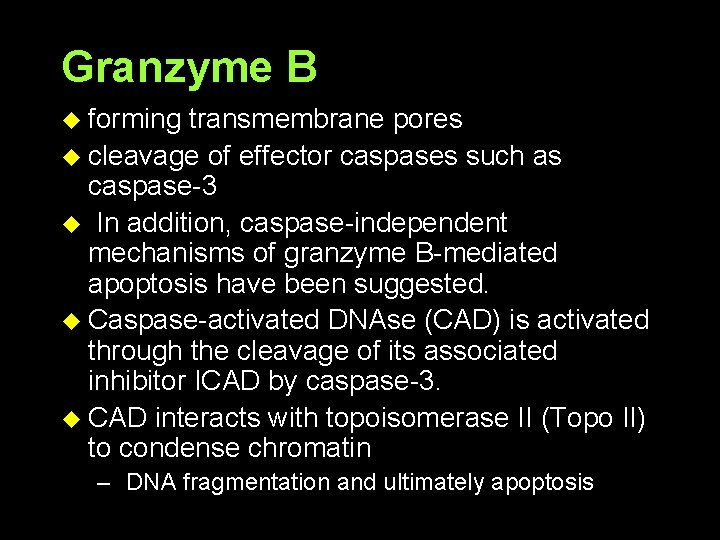
Granzyme B u forming transmembrane pores u cleavage of effector caspases such as caspase-3 u In addition, caspase-independent mechanisms of granzyme B-mediated apoptosis have been suggested. u Caspase-activated DNAse (CAD) is activated through the cleavage of its associated inhibitor ICAD by caspase-3. u CAD interacts with topoisomerase II (Topo II) to condense chromatin – DNA fragmentation and ultimately apoptosis.

Fas Signaling Pathway u u u Fas/APO-1/CD 95 is a member of the tumor necrosis factor (TNF) receptor superfamily – mediator of apoptotic cell death, – involved in inflammation. Binding of the Fas ligand (Fas-L) induces trimerization of Fas in the target cell membrane. Activation of Fas causes the recruitment of Fasassociated protein with death domain (FADD) activation of caspase-8. Activated caspase-8 cleaves (activates) nine other pro-caspases a caspase cascade leads to apoptosis.

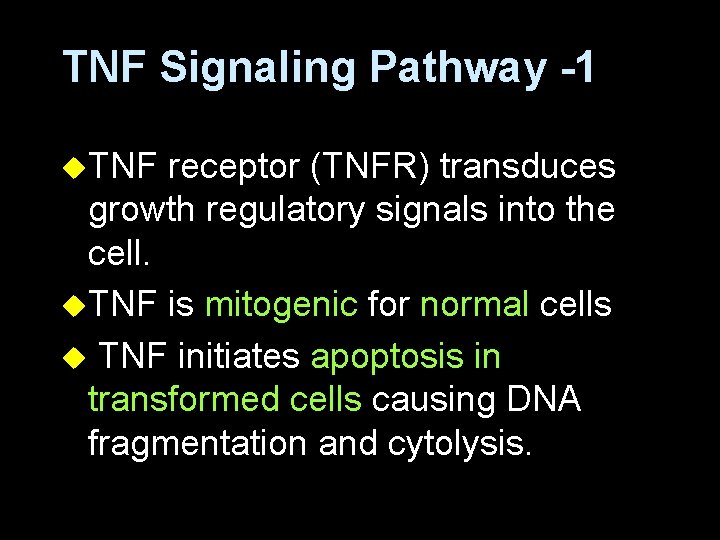
TNF Signaling Pathway -1 u. TNF receptor (TNFR) transduces growth regulatory signals into the cell. u. TNF is mitogenic for normal cells u TNF initiates apoptosis in transformed cells causing DNA fragmentation and cytolysis.

TNF Signaling Pathway - 2 u The TNF-induced cell survival pathway is mediated by the transcription factor NF-k. B. u cells in which the NF-k. B signaling pathway is blocked are more likely to undergo apoptosis in response to TNF. u The availability of NF-k. B may play a critical role in the ability of TNF to act as an apoptosis-inducer and anti-tumor agent.

ATM/p 53 Signaling Pathway u The ataxia telangiectasia-mutated gene (ATM) encodes a protein kinase that acts as a tumor suppressor. u ATM activation stimulates DNA repair and blocks cell cycle progression. u ATM-dependent phosphorylation of p 53 u p 53 can cause growth arrest of the cell at a checkpoint to allow for DNA damage repair or can cause the cell to undergo apoptosis if the damage cannot be repaired. u p 53 is mutated in over 50% of all human cancers.

Integrin Signaling in Cell Survival and Death u Integrins are heterodimeric transmembrane receptors composed of a- and b-subunits. u Approximately 20 integrins have been identified u Focal adhesion kinase (FAK) is activated via autophosphorylation when cells interact through integrins. u Depending on the integrin interactions, the cell can either survive or undergo apoptosis.

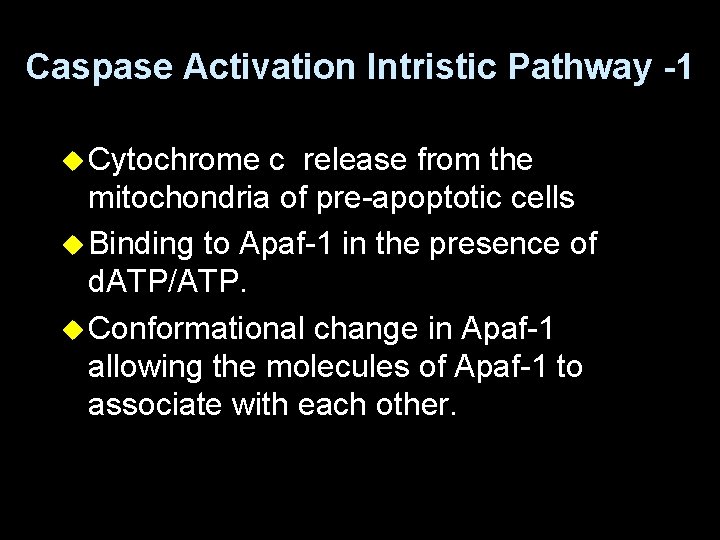
Caspase Activation Intristic Pathway -1 u Cytochrome c release from the mitochondria of pre-apoptotic cells u Binding to Apaf-1 in the presence of d. ATP/ATP. u Conformational change in Apaf-1 allowing the molecules of Apaf-1 to associate with each other.

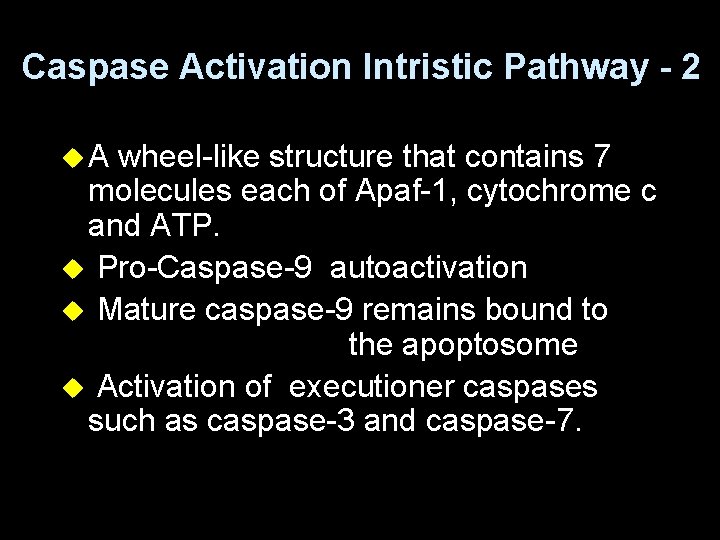
Caspase Activation Intristic Pathway - 2 u. A wheel-like structure that contains 7 molecules each of Apaf-1, cytochrome c and ATP. u Pro-Caspase-9 autoactivation u Mature caspase-9 remains bound to the apoptosome u Activation of executioner caspases such as caspase-3 and caspase-7.

Apoptosis Morphology – chromatin condensation – cell shrinkage – budding and forming of apoptotic bodies (emission of pseudopodia) – karyorrhexis (not pathognomonic for apoptosis)

Apoptosis Meaning physiological process necessary for right organ formatting and life course v pathological process leading to organism damage - e. g. atrophy v

Apoptosis Ontogenesis intestinal mucose, genit. tract, immune system - T lymphocytes Tissue & organ structures turnover intestinal mucose, blood, endometrium Physiological involution Atrophy neonatal adrenal cortex, thymus, breast after lactation period preassure, hyperplasia regression, slight ischemia

Apoptosis Detection v TUNEL = Terminal deoxynukleotidyl transferasemediated d. UTP Nick End Labeling v silver methenamin impregnation

Necrosis Biochemistry – no expression of genes de novo – energy dependent membrane systems damaged hypoxia, toxins – changes in concentrations of ions – increased water volume (oncosis) – autolysis

Necrosis Morphology – pyknosis, karyorhexis, karyolysis – denaturation of proteins - eosinophilia – cell swelling – cell budding (cytoplasmic protrusions)

Necrosis Meaning pathological process leading to a temporary organism damage or death

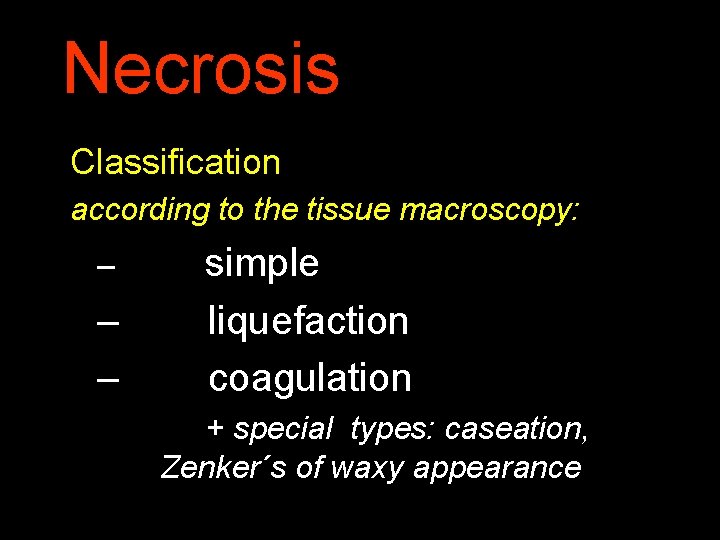
Necrosis Classification according to the tissue macroscopy: – – – simple liquefaction coagulation + special types: caseation, Zenker´s of waxy appearance

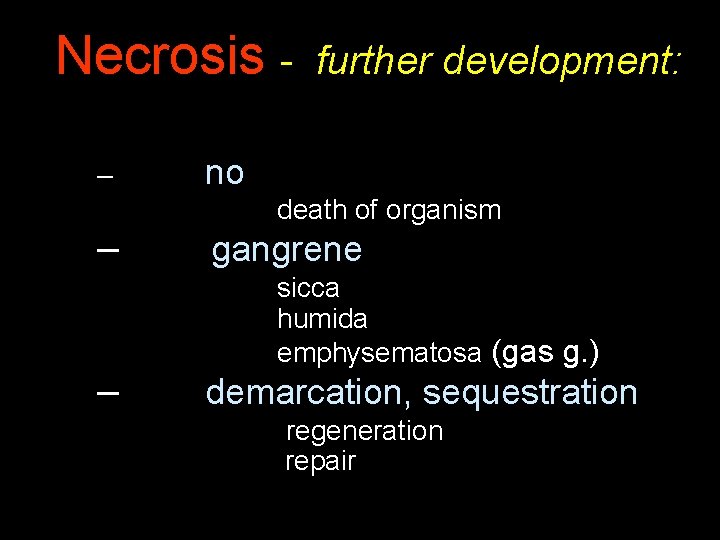
Necrosis – – – further development: no death of organism gangrene sicca humida emphysematosa (gas g. ) demarcation, sequestration regeneration repair

Necrosis u - Causes: chemical – chlorinated hydrocarbons, heavy metal compounds, ethyl- alcohol, aphlatoxins, . . . u physical – mechanical trauma, UV light, ionizing radiation, heat, cold, …. u biologic – bacteria, viruses, fungi. . .

Atrophy diminution of organ or tissue after full development has been attained (versus hypoplasia, aplasia) simple v numerical v (x hypertrophy) (x hyperplasia)

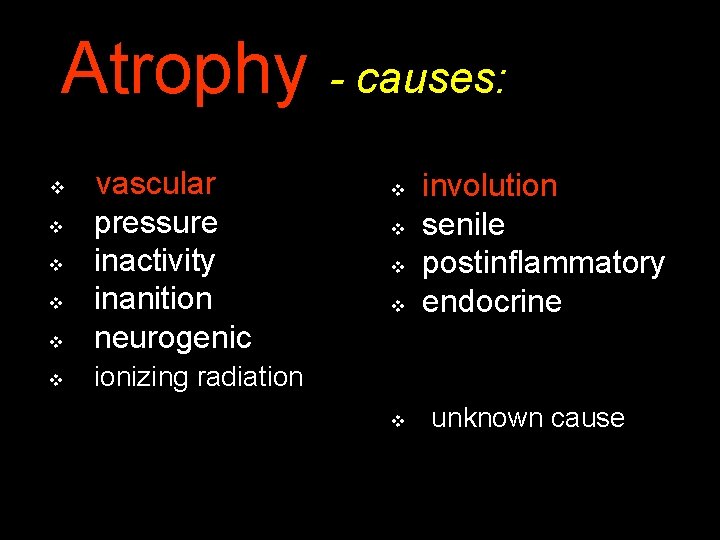
Atrophy - causes: v vascular pressure inactivity inanition neurogenic v ionizing radiation v v v v v involution senile postinflammatory endocrine unknown cause

Atrophy - meaning: v v may be reversible loss of specialised structures & hypofunction clinically silent or unimportant (involution) v clinically apparent v v metaplasia, increase of the supportive tissues - pseudohypertrophy
 Jaroslava healer
Jaroslava healer Mudr. jaroslava orosová ml.
Mudr. jaroslava orosová ml. Opakovanie synonymum
Opakovanie synonymum Aafbr
Aafbr Rubiterm
Rubiterm Hresca inst
Hresca inst Parshvanath charitable trusts a.p.shah inst.of tech
Parshvanath charitable trusts a.p.shah inst.of tech Inst-154
Inst-154 Iata special provision a140
Iata special provision a140 Inst154
Inst154 Inst
Inst Royal inst
Royal inst Inst 301
Inst 301 Inst eecs
Inst eecs Somatic death and molecular death
Somatic death and molecular death A satirical elegy on the death of a late famous general
A satirical elegy on the death of a late famous general Diferencia entre gran plano general y plano general
Diferencia entre gran plano general y plano general Where did general lee surrender to general grant?
Where did general lee surrender to general grant? Domain bacteria characteristics
Domain bacteria characteristics Fungus characteristics
Fungus characteristics Animalia characteristics
Animalia characteristics Biology standards georgia
Biology standards georgia General characteristics of cell
General characteristics of cell Cell city analogy project
Cell city analogy project Advantages of diaphragm cell
Advantages of diaphragm cell Prokaryotic cell vs eukaryotic cell
Prokaryotic cell vs eukaryotic cell Prokaryotic cell and eukaryotic cell similarities
Prokaryotic cell and eukaryotic cell similarities Animal rights vs animal welfare venn diagram
Animal rights vs animal welfare venn diagram What is a half reaction
What is a half reaction Dry cell vs wet cell
Dry cell vs wet cell Animal rights and animal welfare venn diagram
Animal rights and animal welfare venn diagram Cell wall function
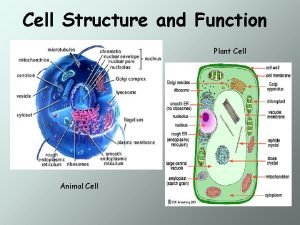
Cell wall function Plant cell and animal cell diagram
Plant cell and animal cell diagram Structure of animalcell
Structure of animalcell Cell wall vs cell membrane
Cell wall vs cell membrane Cell strain
Cell strain