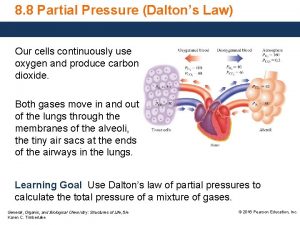
Gases Daltons Law of Partial Pressures Partial Pressure









- Slides: 24

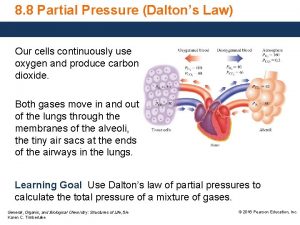
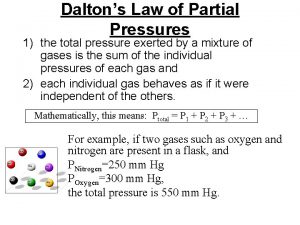
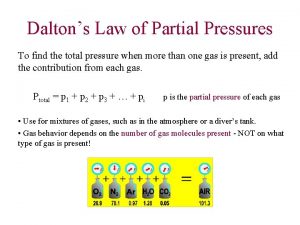
Gases Dalton’s Law of Partial Pressures

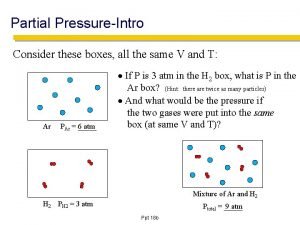
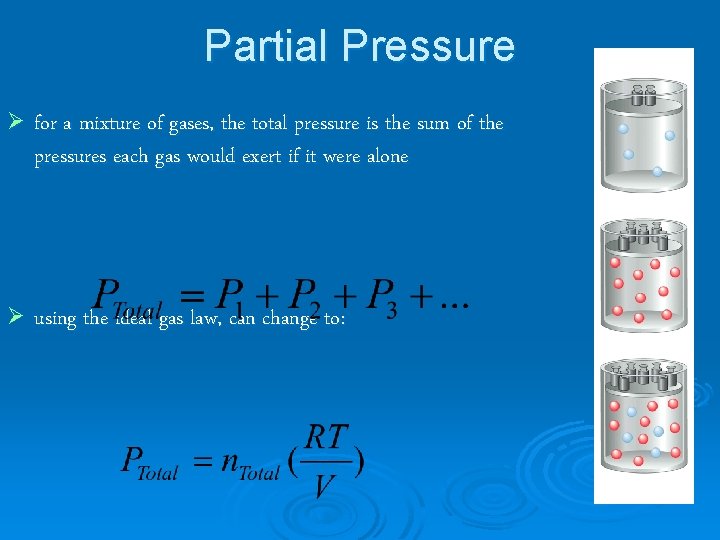
Partial Pressure Ø for a mixture of gases, the total pressure is the sum of the pressures each gas would exert if it were alone Ø using the ideal gas law, can change to:

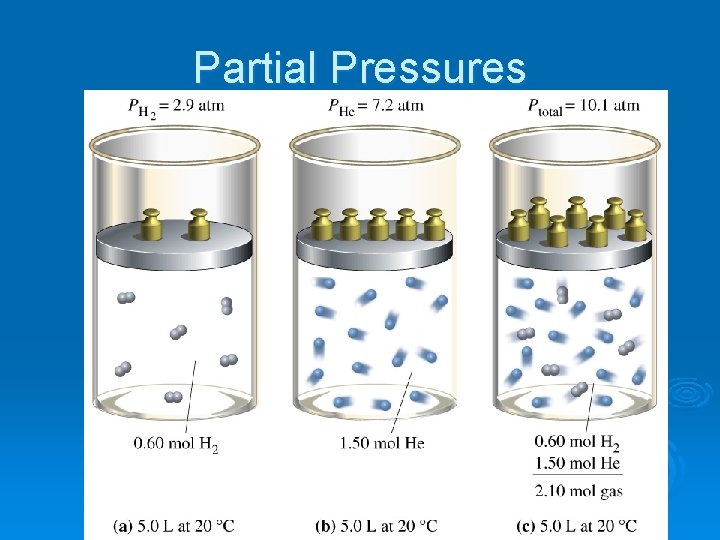
Partial Pressures

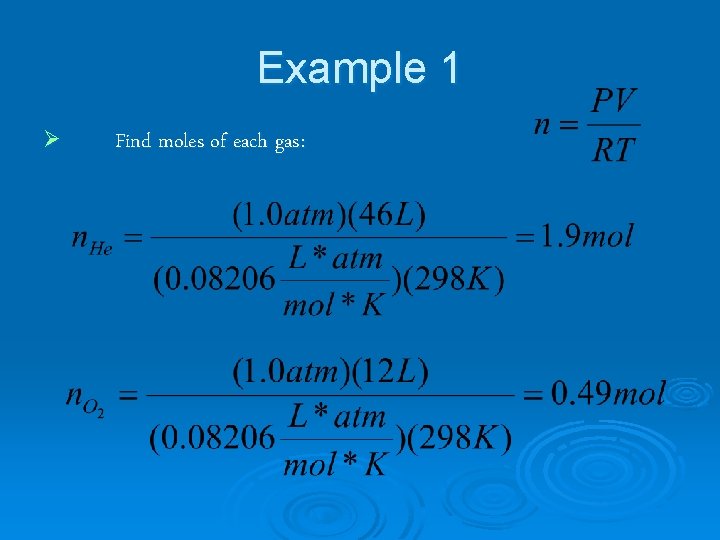
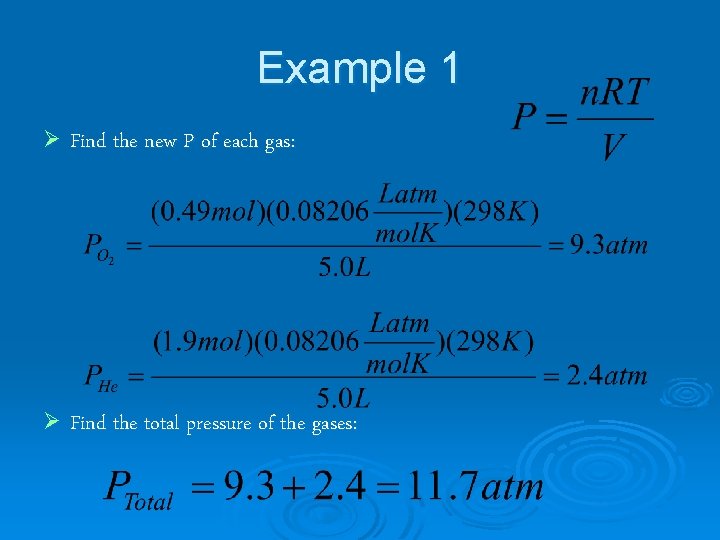
Example 1 47 L He and 12 L O 2 at 25°C and 1. 0 atm were pumped into a tank with a volume of 5. 0 L. Calculate the partial pressure of each gas and the total pressure in the tank.

Example 1 Ø Find moles of each gas:

Example 1 Ø Find the new P of each gas: Ø Find the total pressure of the gases:

Partial Pressure shows that the identities of the gases do not matter, just the number of moles Ø so, for ideal gases: Ø 1. 2. Ø size of gas molecule is not important forces between molecules is not important these are things that would change with the identity of the gas

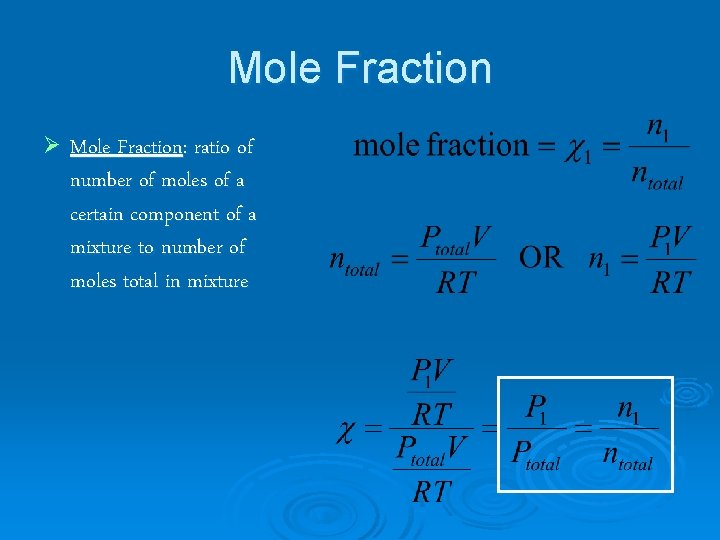
Mole Fraction Ø Mole Fraction: ratio of number of moles of a certain component of a mixture to number of moles total in mixture

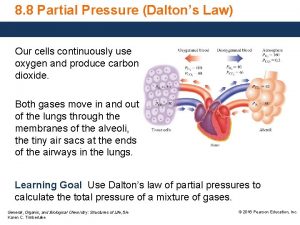
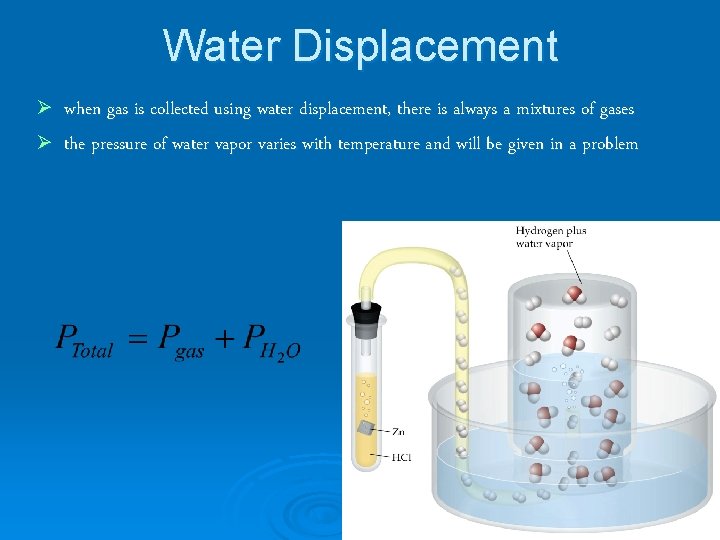
Water Displacement Ø when gas is collected using water displacement, there is always a mixtures of gases Ø the pressure of water vapor varies with temperature and will be given in a problem

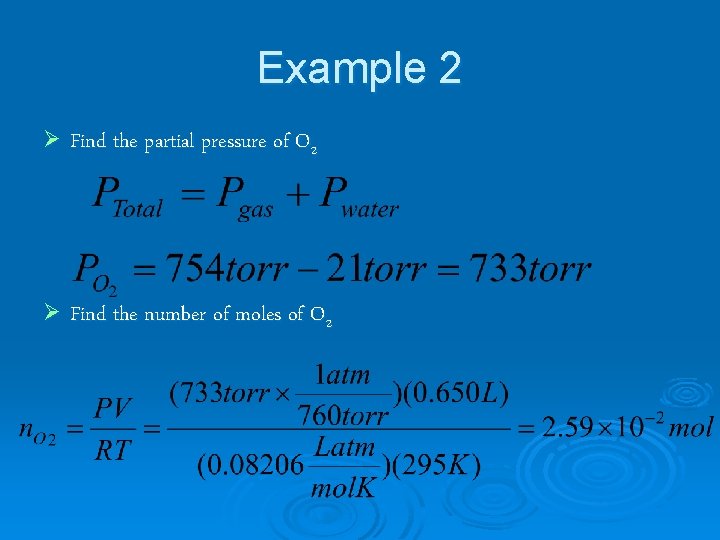
Example 2 The oxygen produced by the reaction below was collected by gas displacement at 22°C at a total pressure of 754 torr. The volume of gas collected was 0. 650 L and the vapor pressure of water at 22°C is 21 torr. Calculate the partial pressure of O 2 in the gas collected and the mass of KCl. O 3 that was decomposed.


Example 2 Ø Find the partial pressure of O 2 Ø Find the number of moles of O 2

Example 2 Ø Find the moles of KCl. O 3 needed: Ø Find the grams of KCl. O 3 needed:

Gases Kinetic Molecular Theory

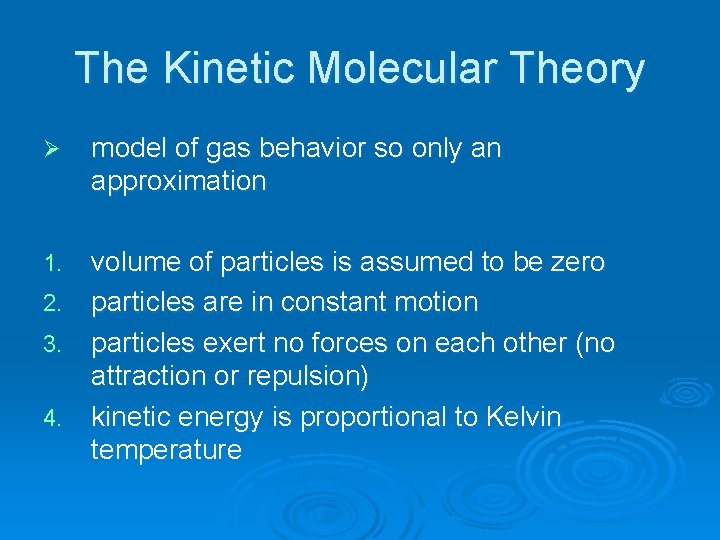
The Kinetic Molecular Theory Ø model of gas behavior so only an approximation volume of particles is assumed to be zero 2. particles are in constant motion 3. particles exert no forces on each other (no attraction or repulsion) 4. kinetic energy is proportional to Kelvin temperature 1.

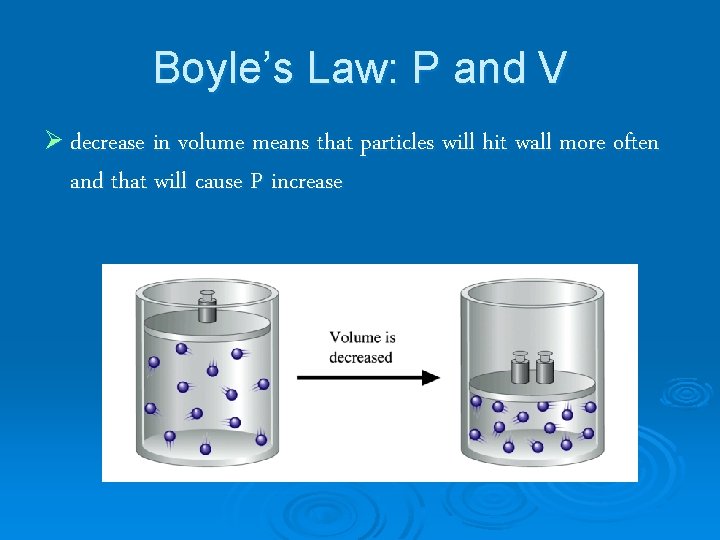
Boyle’s Law: P and V Ø decrease in volume means that particles will hit wall more often and that will cause P increase

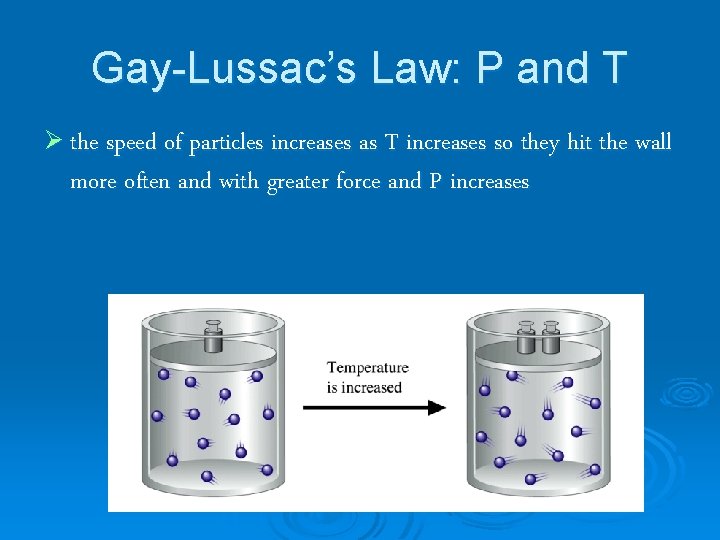
Gay-Lussac’s Law: P and T Ø the speed of particles increases as T increases so they hit the wall more often and with greater force and P increases

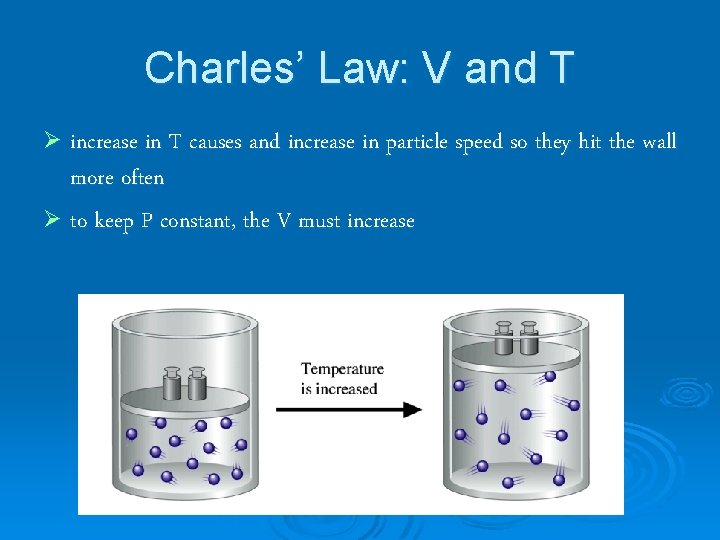
Charles’ Law: V and T Ø increase in T causes and increase in particle speed so they hit the wall more often Ø to keep P constant, the V must increase

Avogadro’s’ Law: V and n Ø increase in number of gas molecules would cause increase in P if V were held constant Ø to keep P constant, V must increase

Dalton’s Law Ø Kinetic Molecular Theory assumes that all particles are independent of each other

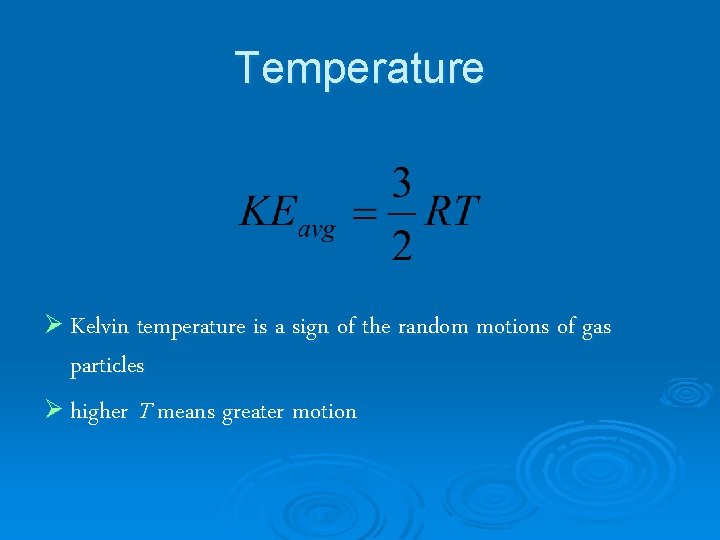
Temperature Ø Kelvin temperature is a sign of the random motions of gas particles Ø higher T means greater motion

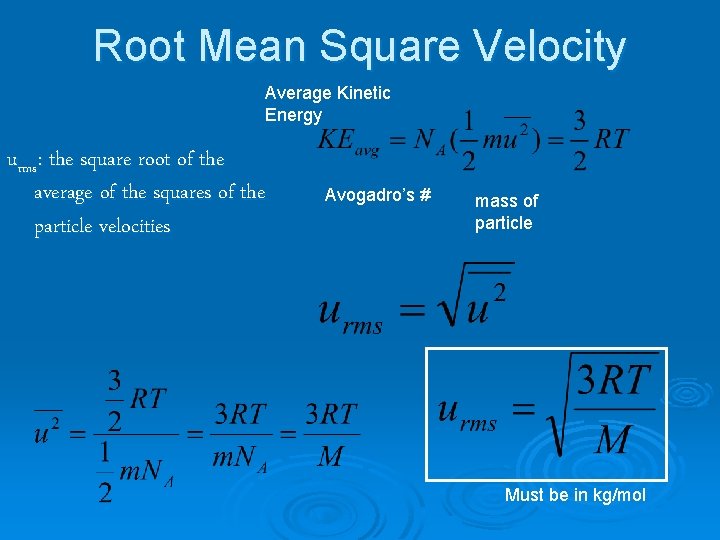
Root Mean Square Velocity Average Kinetic Energy urms: the square root of the average of the squares of the particle velocities Avogadro’s # mass of particle Must be in kg/mol

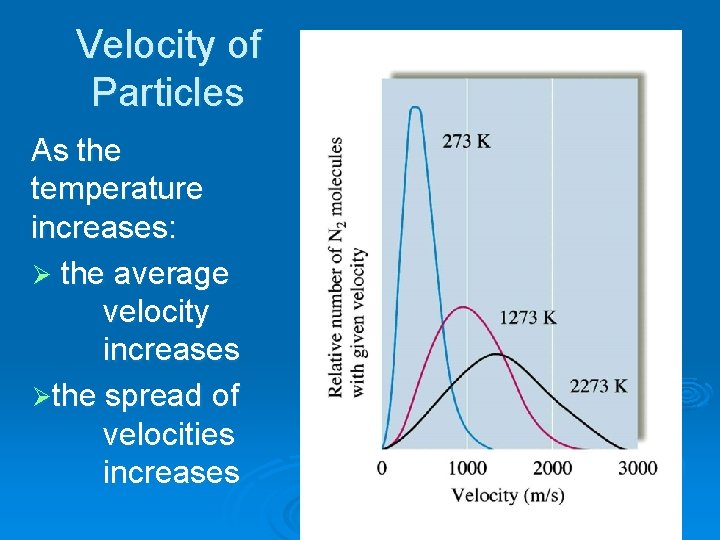
Velocity of Particles As the temperature increases: Ø the average velocity increases Øthe spread of velocities increases

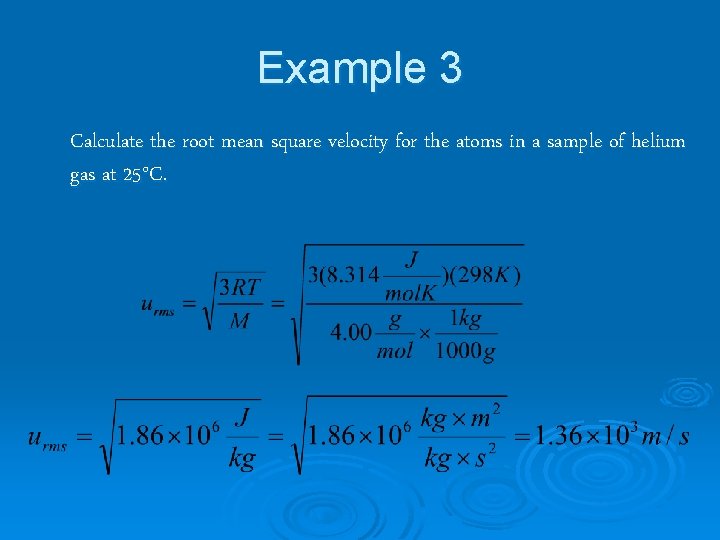
Example 3 Calculate the root mean square velocity for the atoms in a sample of helium gas at 25°C.
 Partial pressures of gases pogil answers
Partial pressures of gases pogil answers Cost pressures and pressures for local responsiveness
Cost pressures and pressures for local responsiveness Ideal gas equation correction
Ideal gas equation correction Two flasks are connected with a stopcock
Two flasks are connected with a stopcock Daltons gas law
Daltons gas law Gibbs free energy
Gibbs free energy Efnisheimurinn kafli 2
Efnisheimurinn kafli 2 John dalton atomic theory
John dalton atomic theory Daltons experiment
Daltons experiment Partial pressure
Partial pressure How to find partial pressure
How to find partial pressure Explain dalton's law of partial pressure
Explain dalton's law of partial pressure How to calculate boyle's law
How to calculate boyle's law Dalton's law
Dalton's law Karen 2
Karen 2 Dalton law of partial pressure
Dalton law of partial pressure Dalton's law of partial pressure
Dalton's law of partial pressure Gas pressure
Gas pressure Do gases exert pressure on whatever surrounds them
Do gases exert pressure on whatever surrounds them How to check superheat
How to check superheat Heart chamber pressures
Heart chamber pressures Chapter 18 handling social pressures
Chapter 18 handling social pressures Which of the following is a pronatalist pressure
Which of the following is a pronatalist pressure Paul trott
Paul trott William zinsser college pressures
William zinsser college pressures