Daltons Law of Partial Pressures Explain how pressure









- Slides: 11

Dalton’s Law of Partial Pressures Explain how pressure, volume and temperature affect the behavior of gases. 1. 2 d

Agenda (4/13) Turn in your Boyle’s law worksheet from Friday for points!! Review gas laws Start partial pressure Take some notes Practice problems Homework

DOL Given MC and CR questions, you will be able to explain how pressure, volume and temperature affect the behavior of gases in real-world situations with 80% accuracy.

Let’s Hypothesize… Think of the gas inside this classroom or the gas that makes up the atmosphere… What is it composed of? Are there multiple gases? By adding the pressures in a container we end up with a total pressure

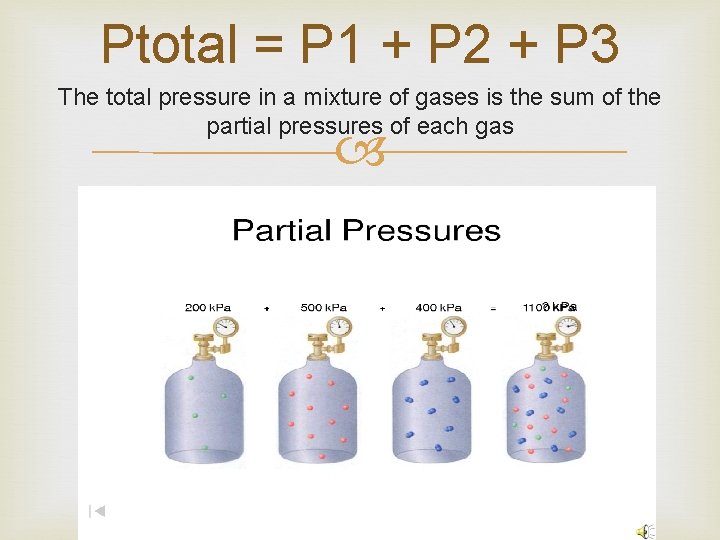
Ptotal = P 1 + P 2 + P 3 The total pressure in a mixture of gases is the sum of the partial pressures of each gas

It’s Solving time! 700 mm. Hg = 1 atm What is the total pressure in a balloon filled with air if the pressure of the oxygen is 170 mm. Hg and the pressure of nitrogen is 620 mm. Hg? 170 + 620 = 790 mm Hg In a second balloon the total pressure is 1. 3 atm. What is the pressure of oxygen if the pressure of nitrogen is 720 mm. Hg? 720 mm. Hg = 1 atm 1. 3 – 1 =. 3 atm

On your own this time In a balloon the total pressure is 4. 0 atm. What is the pressure of oxygen if the pressure of nitrogen is 101. 3 p. Ka? 101. 3 p. Ka = 1 atm 4 – 1 = 3 atm

The gas from an erupting volcano had the following composition: 65. 0 % CO 2, 25. 0 % H 2, 5. 4 % HCl. 2. 8 % HF, 1. 7 % SO 2, and 0. 1 % H 2 S. What are the partial pressures of each gas if the total pressure is 760. 0 mm of Hg? 1. Turn the %’s into decimals 2. Multiply by total pressure. 65(CO 2) +. 25(H 2) +. 054(HCL) +. 028(HF) +. 017(SO 2) +. 001(H 2 S) = P(CO 2) =. 65(760) P(H 2) =. 25(760)

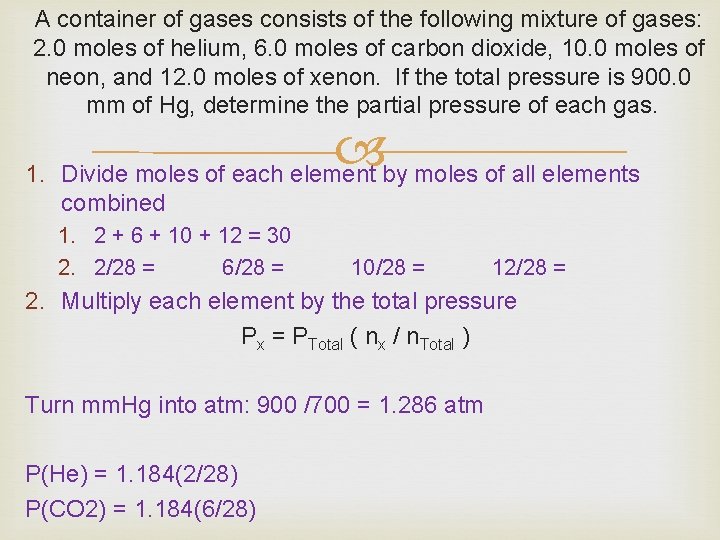
A container of gases consists of the following mixture of gases: 2. 0 moles of helium, 6. 0 moles of carbon dioxide, 10. 0 moles of neon, and 12. 0 moles of xenon. If the total pressure is 900. 0 mm of Hg, determine the partial pressure of each gas. 1. Divide moles of each element by moles of all elements combined 1. 2 + 6 + 10 + 12 = 30 2. 2/28 = 6/28 = 10/28 = 12/28 = 2. Multiply each element by the total pressure Px = PTotal ( nx / n. Total ) Turn mm. Hg into atm: 900 /700 = 1. 286 atm P(He) = 1. 184(2/28) P(CO 2) = 1. 184(6/28)

Your Turn!!! A sealed tank contains the following mixture of gases: 2. 0 grams of helium, 48. 0 grams of oxygen, 14. 0 grams of nitrogen, 64. 0 grams of sulfur (IV) oxide, and 9. 0 grams of hydrogen gas. If the total pressure is 800. 0 k. Pa, then find the partial pressure of each gas. 1. First you must changes them all from g mols 2. Then you must add up all of the moles 3. Then you can solve for each partial pressure using the formula: Px = PTotal ( nx / n. Total )

DOL Put your thinking caps on! Create your own partial pressures problem and make sure you know the correct answer Trade with your neighbor and solve it on a whiteboard Check your answer and trade one more time with someone new Check your answer and clean off your whiteboards