Daltons Partial Pressures Many of the gases around
- Slides: 4

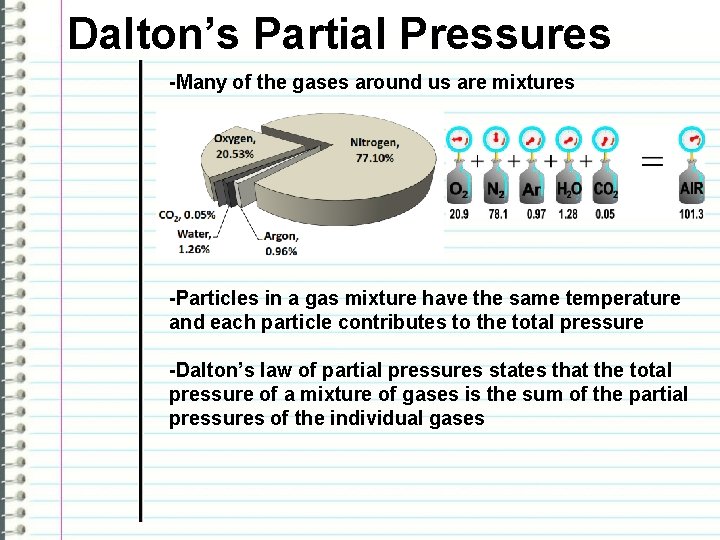
Dalton’s Partial Pressures -Many of the gases around us are mixtures -Particles in a gas mixture have the same temperature and each particle contributes to the total pressure -Dalton’s law of partial pressures states that the total pressure of a mixture of gases is the sum of the partial pressures of the individual gases

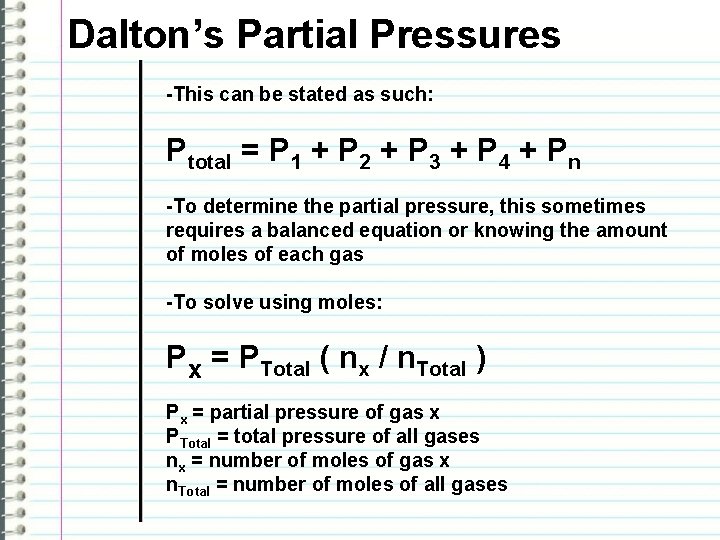
Dalton’s Partial Pressures -This can be stated as such: Ptotal = P 1 + P 2 + P 3 + P 4 + Pn -To determine the partial pressure, this sometimes requires a balanced equation or knowing the amount of moles of each gas -To solve using moles: Px = PTotal ( nx / n. Total ) Px = partial pressure of gas x PTotal = total pressure of all gases nx = number of moles of gas x n. Total = number of moles of all gases

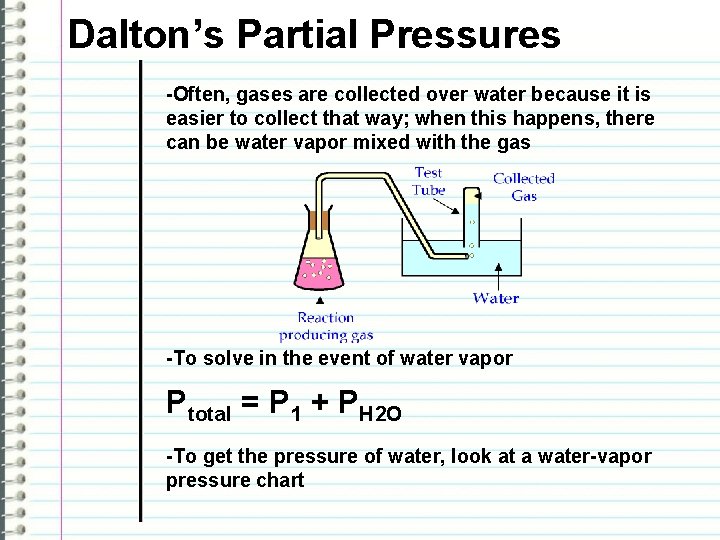
Dalton’s Partial Pressures -Often, gases are collected over water because it is easier to collect that way; when this happens, there can be water vapor mixed with the gas -To solve in the event of water vapor Ptotal = P 1 + PH 2 O -To get the pressure of water, look at a water-vapor pressure chart

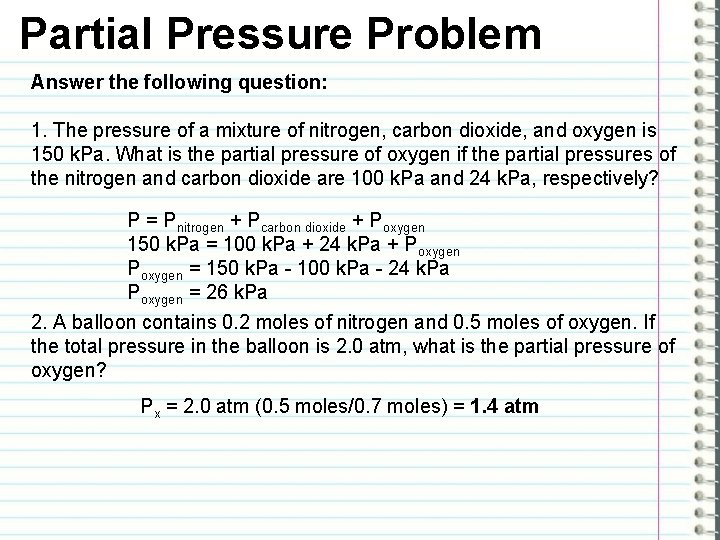
Partial Pressure Problem Answer the following question: 1. The pressure of a mixture of nitrogen, carbon dioxide, and oxygen is 150 k. Pa. What is the partial pressure of oxygen if the partial pressures of the nitrogen and carbon dioxide are 100 k. Pa and 24 k. Pa, respectively? P = Pnitrogen + Pcarbon dioxide + Poxygen 150 k. Pa = 100 k. Pa + 24 k. Pa + Poxygen = 150 k. Pa - 100 k. Pa - 24 k. Pa Poxygen = 26 k. Pa 2. A balloon contains 0. 2 moles of nitrogen and 0. 5 moles of oxygen. If the total pressure in the balloon is 2. 0 atm, what is the partial pressure of oxygen? Px = 2. 0 atm (0. 5 moles/0. 7 moles) = 1. 4 atm