Chapter 6 Gases 6 8 Partial Pressure Daltons





- Slides: 17

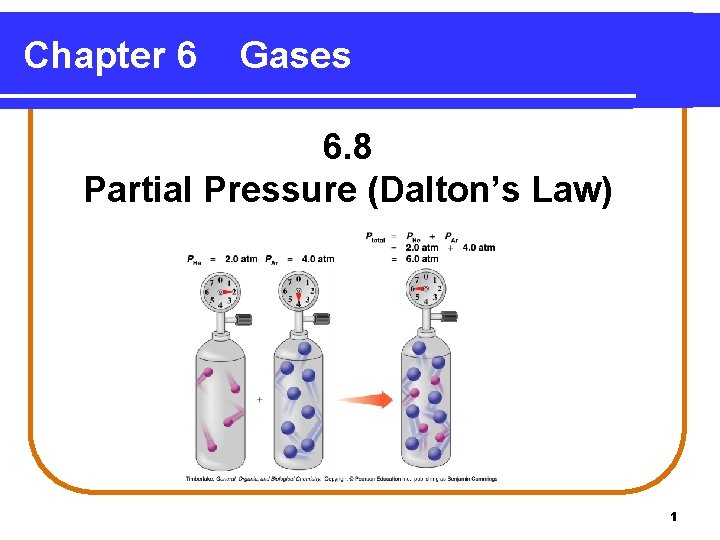
Chapter 6 Gases 6. 8 Partial Pressure (Dalton’s Law) 1

Partial Pressure The partial pressure of a gas • is the pressure of each gas in a mixture. • is the pressure that gas would exert if it were by itself in the container. 2

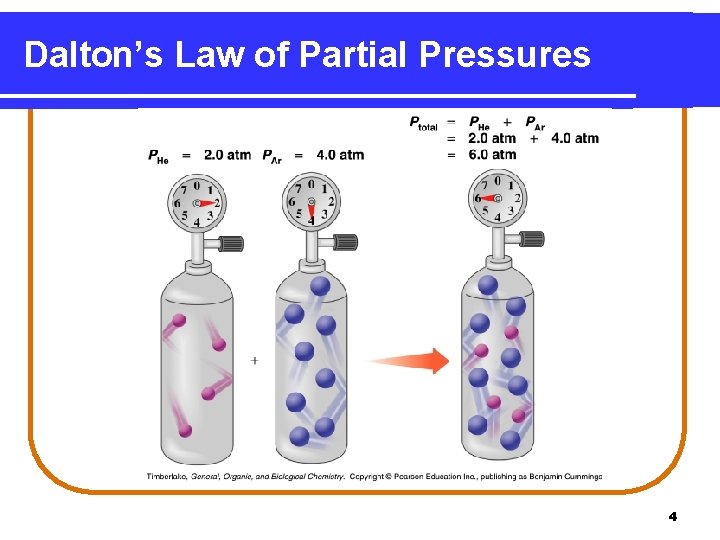
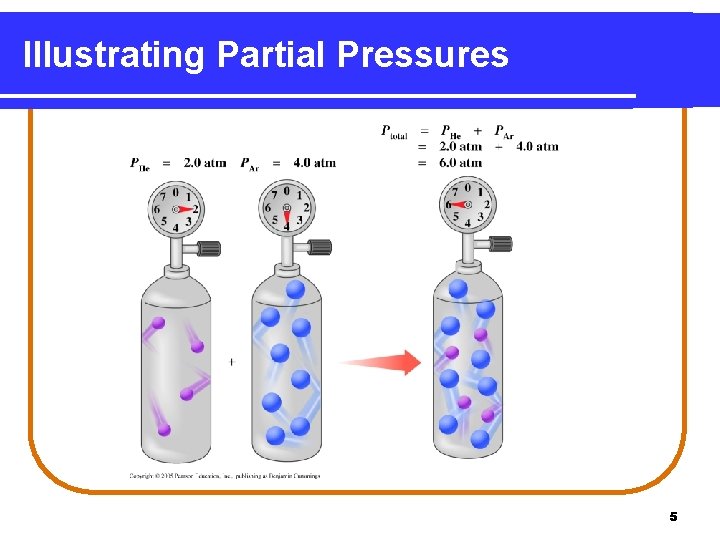
Dalton’s Law of Partial Pressures indicates that • pressure depends on the total number of gas particles, not on the types of particles. • the total pressure exerted by gases in a mixture is the sum of the partial pressures of those gases. PT = P 1 + P 2 + P 3 +. . . 3

Dalton’s Law of Partial Pressures 4

Illustrating Partial Pressures 5

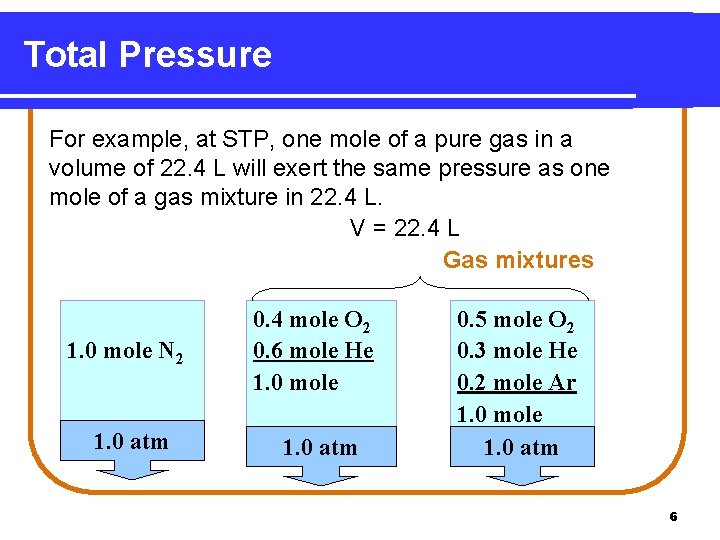
Total Pressure For example, at STP, one mole of a pure gas in a volume of 22. 4 L will exert the same pressure as one mole of a gas mixture in 22. 4 L. V = 22. 4 L Gas mixtures 1. 0 mole N 2 1. 0 atm 0. 4 mole O 2 0. 6 mole He 1. 0 mole 1. 0 atm 0. 5 mole O 2 0. 3 mole He 0. 2 mole Ar 1. 0 mole 1. 0 atm 6

Scuba Diving • When a scuba diver dives, the increased pressure causes N 2(g) to dissolve in the blood. • If a diver rises too fast, the dissolved N 2 will form bubbles in the blood, a dangerous and painful condition called "the bends". • Helium, which does not dissolve in the blood, is mixed with O 2 to prepare breathing mixtures for deep descents. 7

Learning Check A scuba tank contains O 2 with a pressure of 0. 450 atm and He at 855 mm Hg. What is the total pressure in mm Hg in the tank? 8

Solution 1. Convert the pressure in atm to mm Hg 0. 450 atm x 760 mm Hg = 342 mm Hg = PO 2 1 atm 2. Calculate the sum of the partial pressures. Ptotal = PO 2 + PHe Ptotal = 342 mm Hg + 855 mm Hg = 1197 mm Hg 9

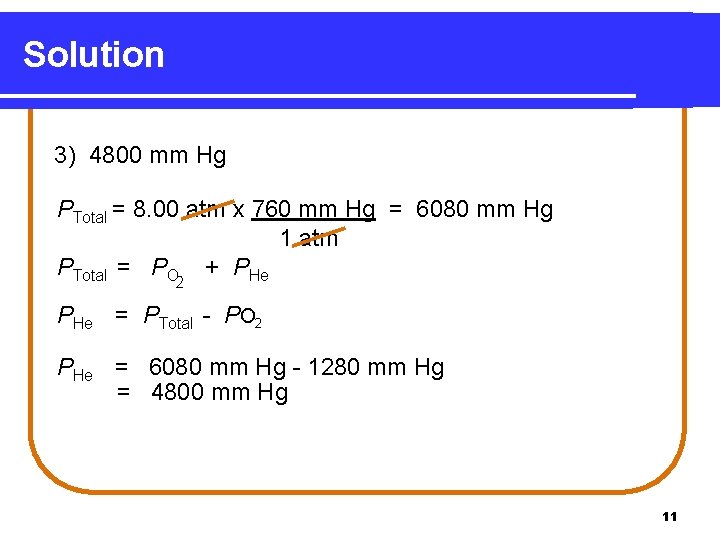
Learning Check For a deep dive, a scuba diver uses a mixture of helium and oxygen with a pressure of 8. 00 atm. If the oxygen has a partial pressure of 1280 mm Hg, what is the partial pressure of the helium? 1) 520 mm Hg 2) 2040 mm Hg 3) 4800 mm Hg 10

Solution 3) 4800 mm Hg PTotal = 8. 00 atm x 760 mm Hg = 6080 mm Hg 1 atm PTotal = PO + PHe 2 PHe = PTotal - PO 2 PHe = 6080 mm Hg - 1280 mm Hg = 4800 mm Hg 11

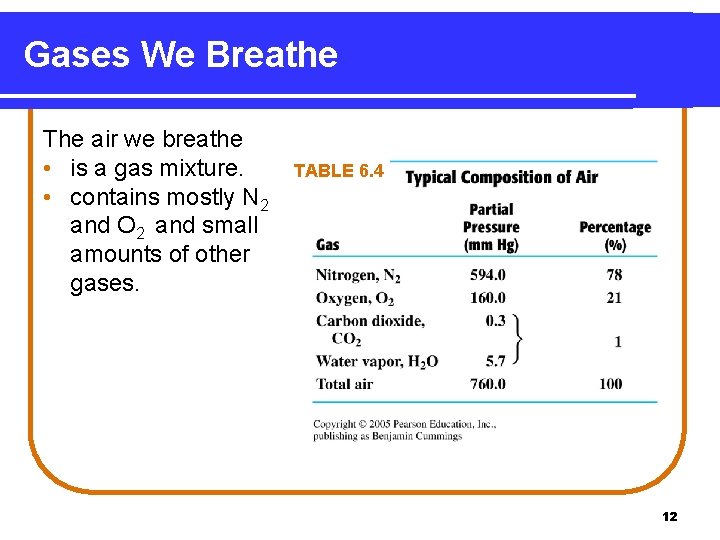
Gases We Breathe The air we breathe • is a gas mixture. • contains mostly N 2 and O 2 and small amounts of other gases. TABLE 6. 4 12

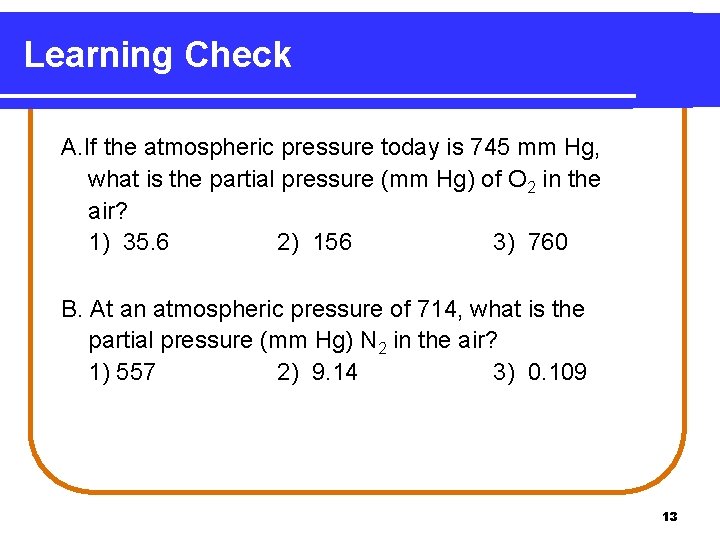
Learning Check A. If the atmospheric pressure today is 745 mm Hg, what is the partial pressure (mm Hg) of O 2 in the air? 1) 35. 6 2) 156 3) 760 B. At an atmospheric pressure of 714, what is the partial pressure (mm Hg) N 2 in the air? 1) 557 2) 9. 14 3) 0. 109 13

Solution A. If the atmospheric pressure today is 745 mm Hg, what is the partial pressure (mm Hg) of O 2 in the air? 2) 156 B. At an atmospheric pressure of 714, what is the partial pressure (mm Hg) N 2 in the air? 1) 557 14

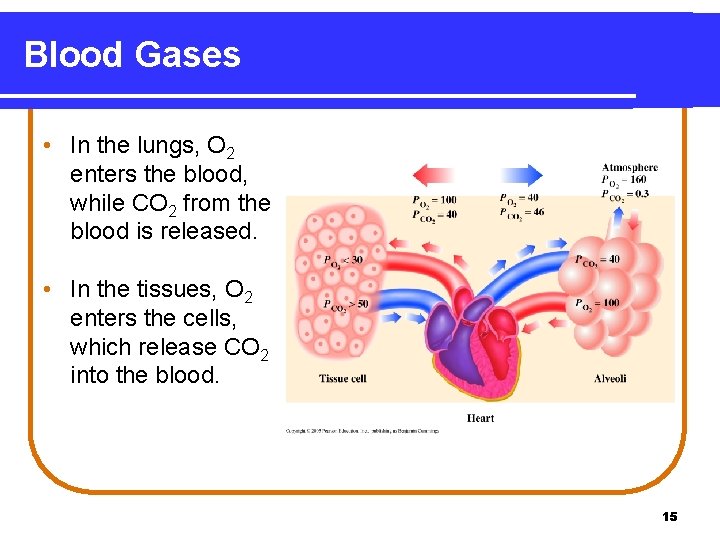
Blood Gases • In the lungs, O 2 enters the blood, while CO 2 from the blood is released. • In the tissues, O 2 enters the cells, which release CO 2 into the blood. 15

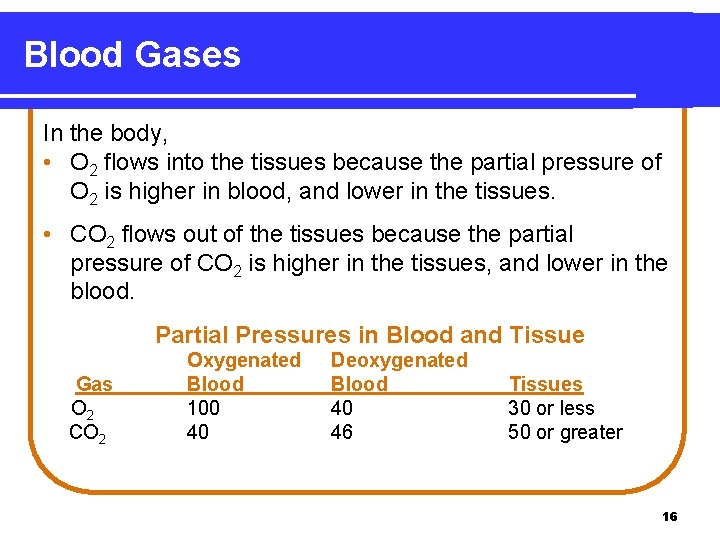
Blood Gases In the body, • O 2 flows into the tissues because the partial pressure of O 2 is higher in blood, and lower in the tissues. • CO 2 flows out of the tissues because the partial pressure of CO 2 is higher in the tissues, and lower in the blood. Partial Pressures in Blood and Tissue Gas O 2 CO 2 Oxygenated Blood 100 40 Deoxygenated Blood 40 46 Tissues 30 or less 50 or greater 16

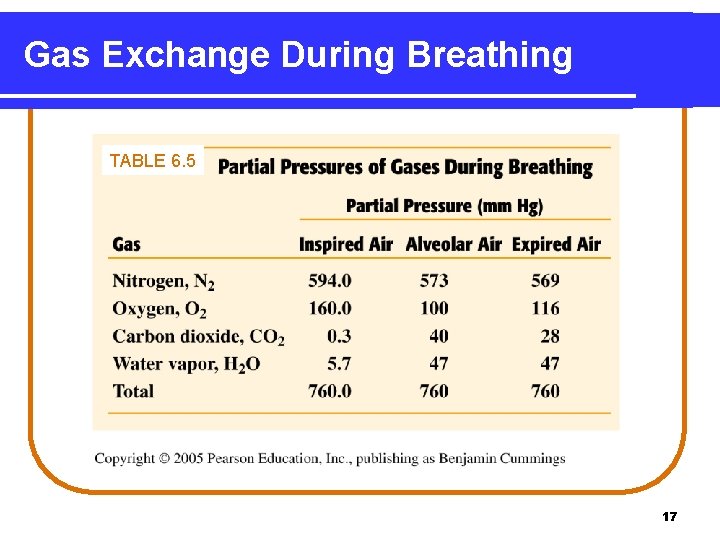
Gas Exchange During Breathing TABLE 6. 5 17