Gases cont and finished Ideal vs Real Ideal












- Slides: 17

Gases, cont. (and finished!) Ideal vs. Real Ideal Gas Law Dalton’s Law Diffusion

Chemistry Joke A Chemistry Prison Punsilicon put his neon The the window ledge. He climbed out and then krypton along the wall to meet his buddy. I hope the guard cesium

Ideal vs. Real b Ideal Gases • Follow gas laws at all conditions • Conform to the Kinetic Molecular Theory Ø Insignificant volume Ø No attraction / repulsion to each other

Ideal vs. Real b Ideal Gases don’t exist!!! • Molecules do take up space • There attractive forces between them Ø Otherwise gases would never liquefy • But…at many temperatures and pressures real gases act like ideal gases. • This makes for easier math!

Ideal vs. Real b Real Gases behave ideally… • At HIGH TEMPERATURES and LOW PRESSURES… Ø In these conditions, a gas will stay a gas. Ø At low pressures, the molecules are far apart. –We can ignore their volume. Ø At high temperatures, molecules are not together very long. –We can ignore their attractions.

Ideal vs. Real • At LOW TEMPERATURES and HIGH PRESSURES… Ø Gases experience both particle volume and attraction Ø Can be liquefied or solidified –Think of Dry Ice b We’ll continue to study ideal gases!

Ideal Gas Law b PV = n. RT n represents number of moles b R is the universal gas constant b

Ideal Gas Law b. Units for P and V have to match “R”

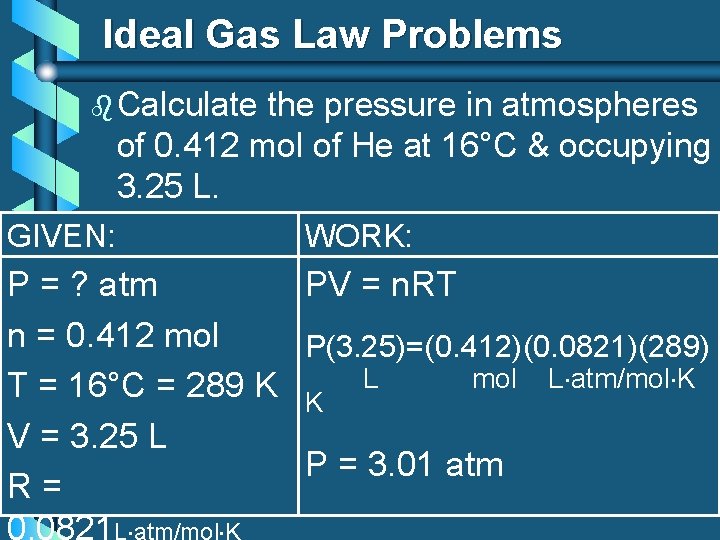
Ideal Gas Law Problems b Calculate the pressure in atmospheres of 0. 412 mol of He at 16°C & occupying 3. 25 L. GIVEN: WORK: P = ? atm PV = n. RT n = 0. 412 mol P(3. 25)=(0. 412)(0. 0821)(289) mol L atm/mol K T = 16°C = 289 K K L V = 3. 25 L P = 3. 01 atm R= 0. 0821 L atm/mol K

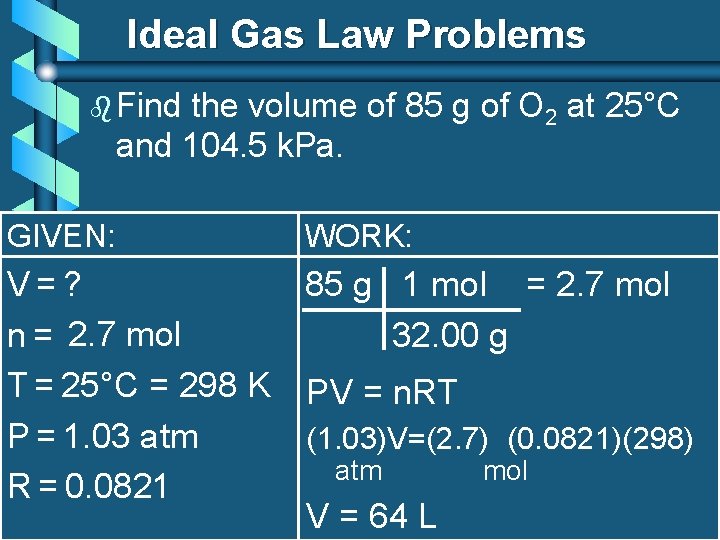
Ideal Gas Law Problems b Find the volume of 85 g of O 2 at 25°C and 104. 5 k. Pa. GIVEN: WORK: V=? 85 g 1 mol = 2. 7 mol n = 2. 7 mol 32. 00 g T = 25°C = 298 K PV = n. RT P = 1. 03 atm (1. 03)V=(2. 7) (0. 0821)(298) atm mol R = 0. 0821 V = 64 L

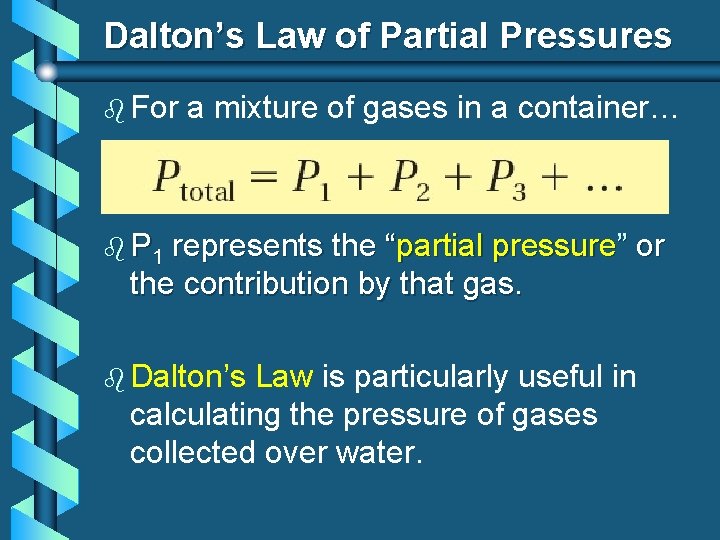
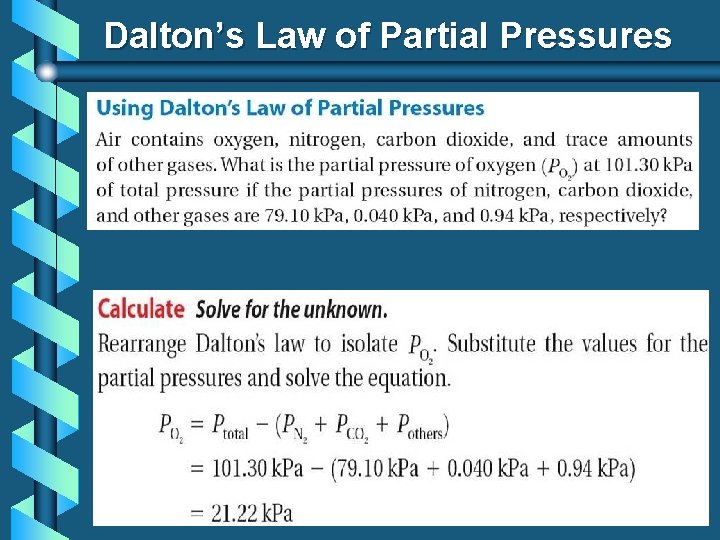
Dalton’s Law of Partial Pressures b For a mixture of gases in a container… b P 1 represents the “partial pressure” or the contribution by that gas. b Dalton’s Law is particularly useful in calculating the pressure of gases collected over water.

Dalton’s Law of Partial Pressures b If the gases in the first three containers are all put into the fourth, we can find the pressure in the 4 th container by adding up the pressures in the first 3:

Dalton’s Law of Partial Pressures

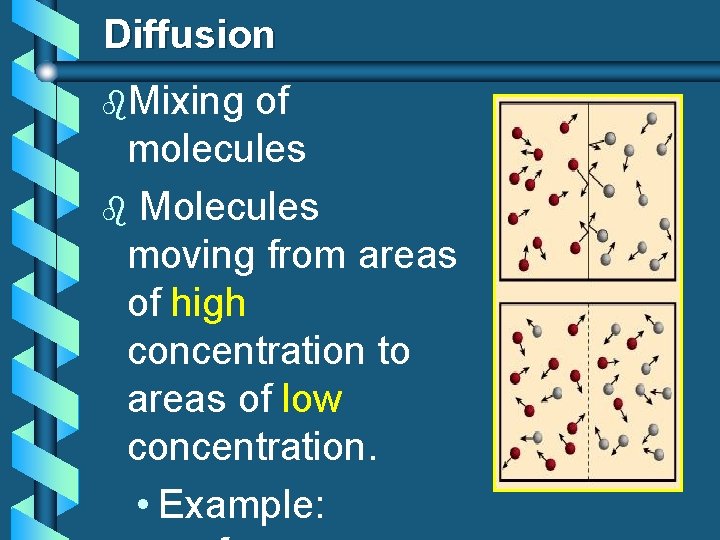
Diffusion b. Mixing of molecules b Molecules moving from areas of high concentration to areas of low concentration. • Example:

Diffusion b. With diffusion, the mass of the particle is important: • Gases of lower molar mass diffuse faster than gases of higher molar mass.

Diffusion b. Example: compare diffusion rates of helium and nitrogen. b. The molar mass of He = 4. 0 g b. The molar mass of N 2 = 28. 02 g • Therefore… b. Helium diffuses faster than nitrogen.

Chemistry Joke Q: What did the electron say to the proton to make it unhappy? A: Something negative!
 Cont or cont'd
Cont or cont'd Cont or cont'd
Cont or cont'd Kinetic molecular theory of gases
Kinetic molecular theory of gases Characteristics of ideal gases
Characteristics of ideal gases Characteristics of ideal gas
Characteristics of ideal gas Compressibility of solid liquid and gas
Compressibility of solid liquid and gas First law of thermodynamics for ideal gas
First law of thermodynamics for ideal gas For real gases specific heat
For real gases specific heat Fugacity of real gases
Fugacity of real gases Della finished her cry and attended
Della finished her cry and attended Future plans and finished future actions
Future plans and finished future actions True self and ideal self
True self and ideal self When did you finish your homework
When did you finish your homework Final sketch
Final sketch 21cfr211.170
21cfr211.170 I have just finished an absolutely
I have just finished an absolutely John 19:25-30
John 19:25-30 Etiquette silverware placement when finished
Etiquette silverware placement when finished