FXR agonist in NASH 2019 04 04 Pf







- Slides: 41

FXR agonist in NASH 2019년 04월 04일 목요세미나 Pf 김 원 / R 4 이철형

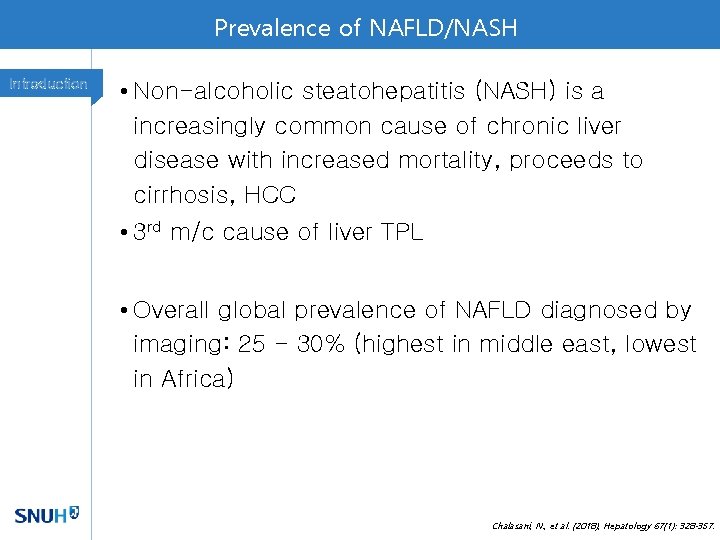
Prevalence of NAFLD/NASH Introduction • Non-alcoholic steatohepatitis (NASH) is a increasingly common cause of chronic liver disease with increased mortality, proceeds to cirrhosis, HCC • 3 rd m/c cause of liver TPL • Overall global prevalence of NAFLD diagnosed by imaging: 25 - 30% (highest in middle east, lowest in Africa) Chalasani, N. , et al. (2018), Hepatology 67(1): 328 -357.

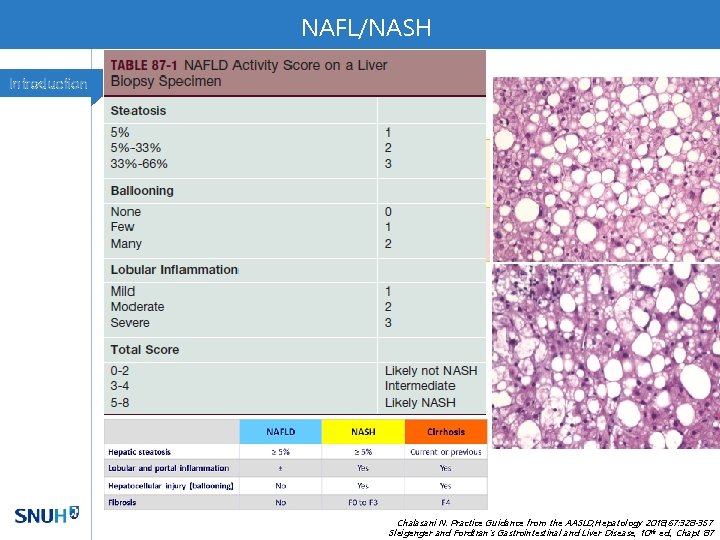
NAFL/NASH Introduction Chalasani N. Practice Guidance from the AASLD; Hepatology 2018; 67: 328 -357 Sleigenger and Fordtran's Gastrointestinal and Liver Disease, 10 th ed. , Chapt 87

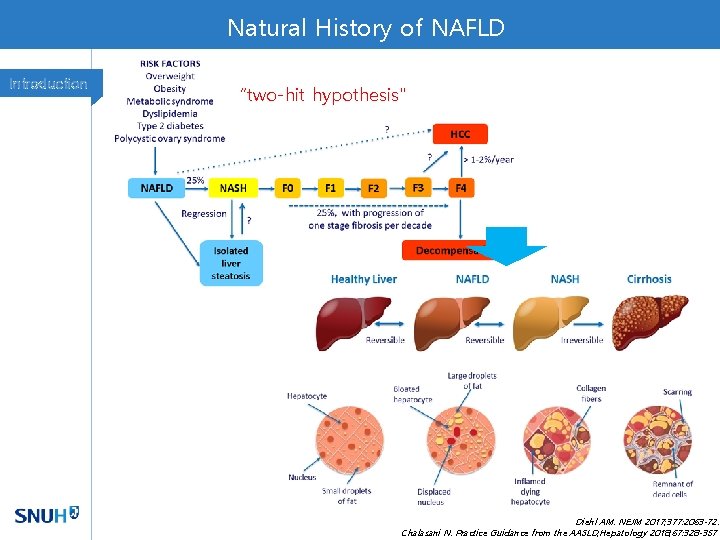
Natural History of NAFLD Introduction “two-hit hypothesis" Diehl AM. NEJM 2017; 377: 2063 -72. Chalasani N. Practice Guidance from the AASLD; Hepatology 2018; 67: 328 -357

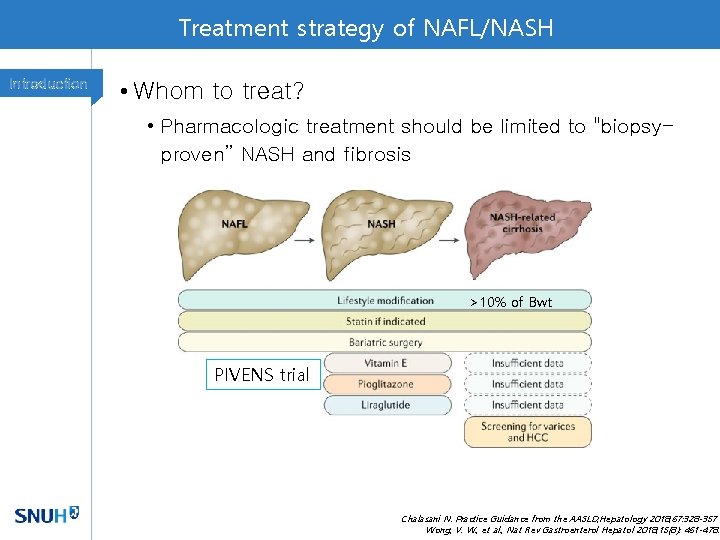
Treatment strategy of NAFL/NASH Introduction • Whom to treat? • Pharmacologic treatment should be limited to "biopsyproven” NASH and fibrosis >10% of Bwt PIVENS trial Chalasani N. Practice Guidance from the AASLD; Hepatology 2018; 67: 328 -357 Wong, V. W. , et al. , Nat Rev Gastroenterol Hepatol 2018; 15(8): 461 -478.

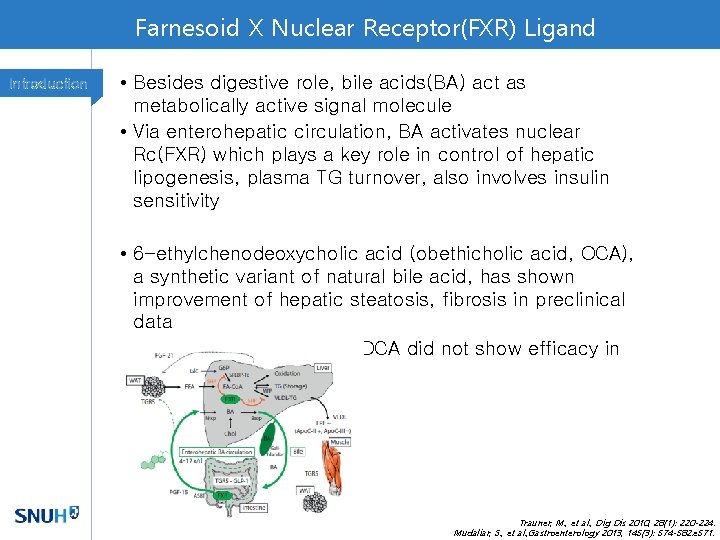
Farnesoid X Nuclear Receptor(FXR) Ligand Introduction • Besides digestive role, bile acids(BA) act as metabolically active signal molecule • Via enterohepatic circulation, BA activates nuclear Rc(FXR) which plays a key role in control of hepatic lipogenesis, plasma TG turnover, also involves insulin sensitivity • 6 -ethylchenodeoxycholic acid (obethicholic acid, OCA), a synthetic variant of natural bile acid, has shown improvement of hepatic steatosis, fibrosis in preclinical data • Less lipophilic bile acid UDCA did not show efficacy in clinical trials Trauner, M. , et al. , Dig Dis 2010, 28(1): 220 -224. Mudaliar, S. , et al. , Gastroenterology 2013, 145(3): 574 -582. e 571.


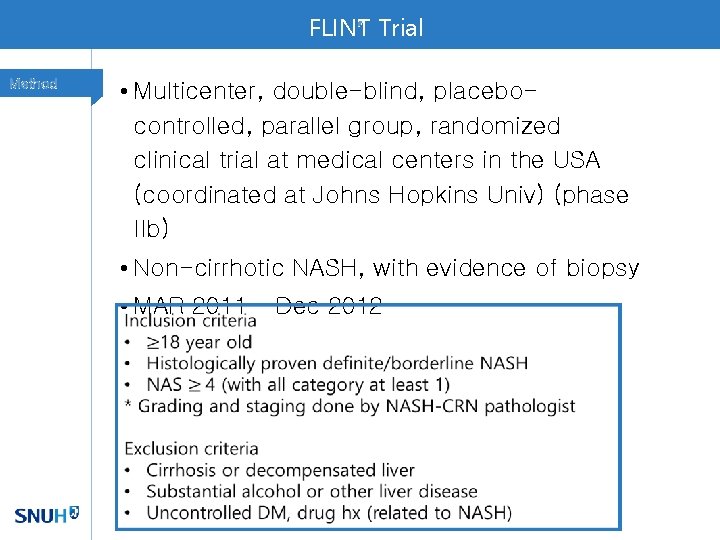
FLINT Trial F Method • Multicenter, double-blind, placebocontrolled, parallel group, randomized clinical trial at medical centers in the USA (coordinated at Johns Hopkins Univ) (phase IIb) • Non-cirrhotic NASH, with evidence of biopsy • MAR 2011 – Dec 2012

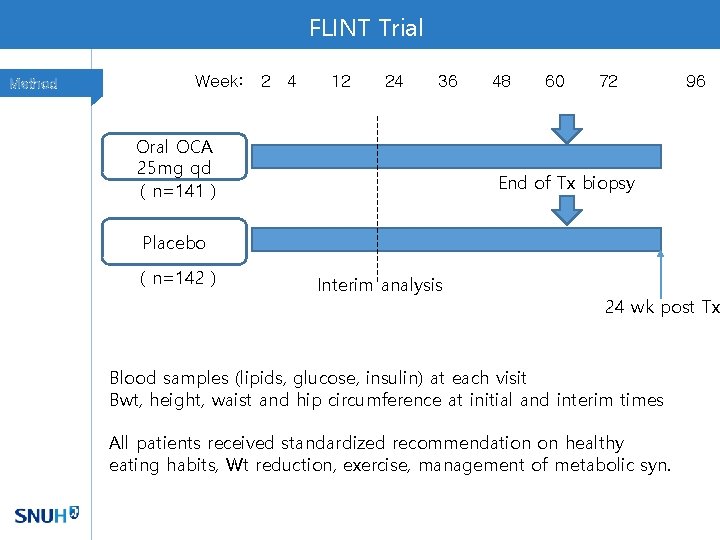
FLINT Trial Method Week: 2 4 12 24 36 Oral OCA 25 mg qd ( n=141 ) 48 60 72 96 End of Tx biopsy Placebo ( n=142 ) Interim analysis 24 wk post Tx Blood samples (lipids, glucose, insulin) at each visit Bwt, height, waist and hip circumference at initial and interim times All patients received standardized recommendation on healthy eating habits, Wt reduction, exercise, management of metabolic syn.

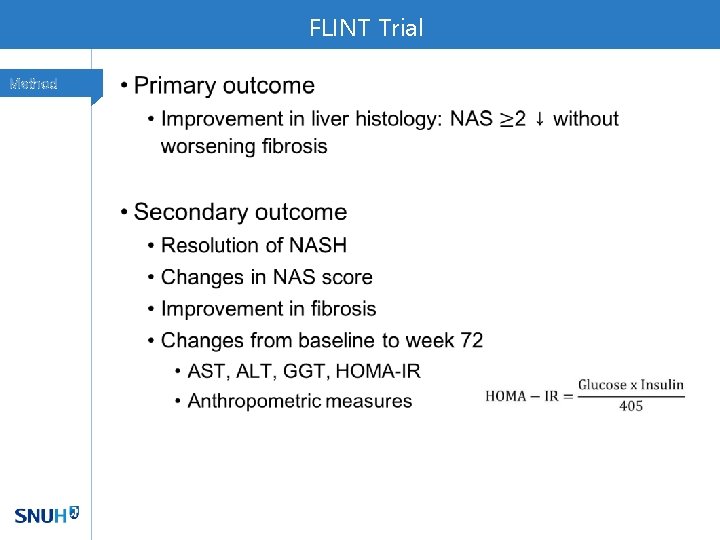
FLINT Trial Method •

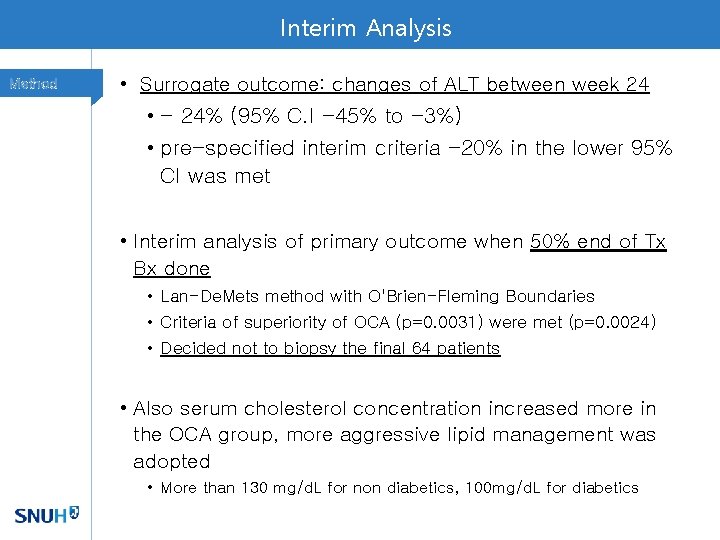
Interim Analysis Method • Surrogate outcome: changes of ALT between week 24 • - 24% (95% C. I -45% to -3%) • pre-specified interim criteria -20% in the lower 95% CI was met • Interim analysis of primary outcome when 50% end of Tx Bx done • Lan-De. Mets method with O'Brien-Fleming Boundaries • Criteria of superiority of OCA (p=0. 0031) were met (p=0. 0024) • Decided not to biopsy the final 64 patients • Also serum cholesterol concentration increased more in the OCA group, more aggressive lipid management was adopted • More than 130 mg/d. L for non diabetics, 100 mg/d. L for diabetics

Statistical Analysis Method • Intention-to-treat analysis for primary outcome excluded 64 patients (without Bx) • All patients included secondary non-histological outcomes • Stratified by clinical center and diabetes status • Primary and binary secondary outcomes • Mantel-Haenszel test • Continuous secondary outcomes from baseline to 72 weeks • ANCOVA models

Trial Profile Result

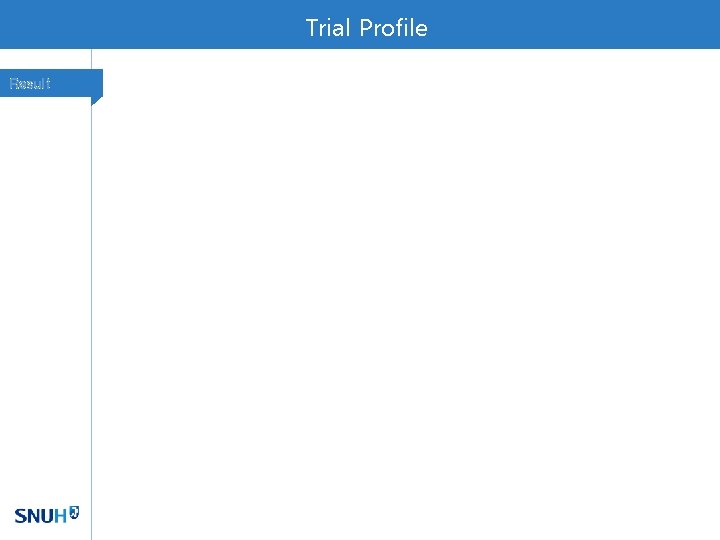
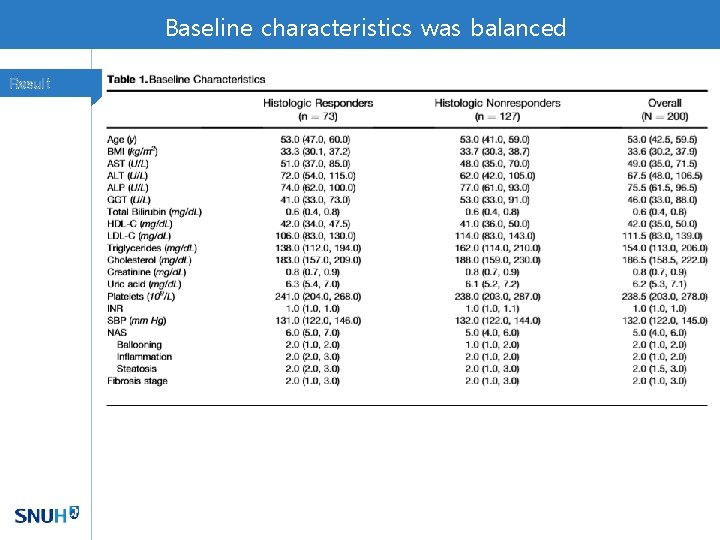
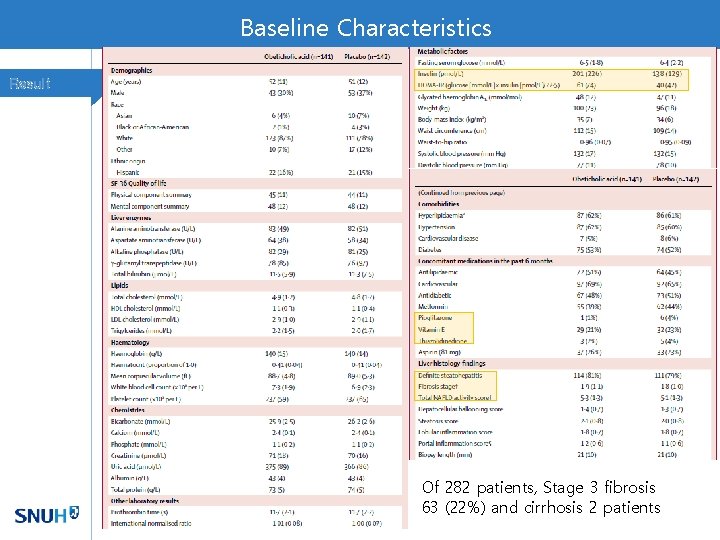
Baseline Characteristics Result Of 282 patients, Stage 3 fibrosis 63 (22%) and cirrhosis 2 patients

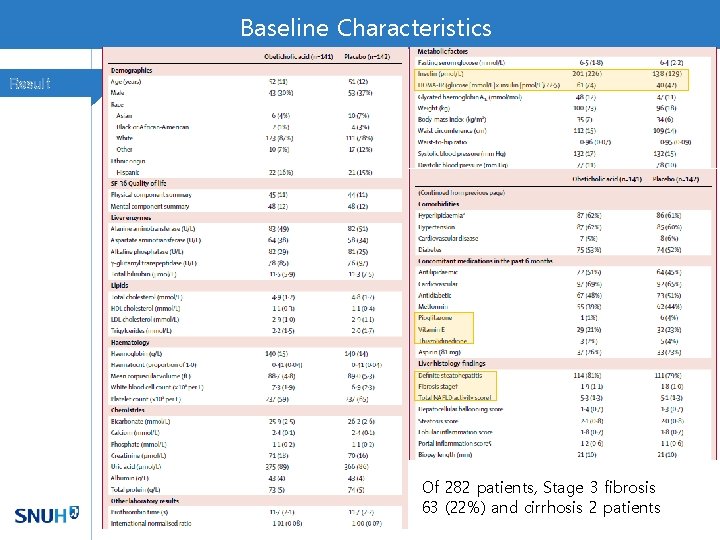
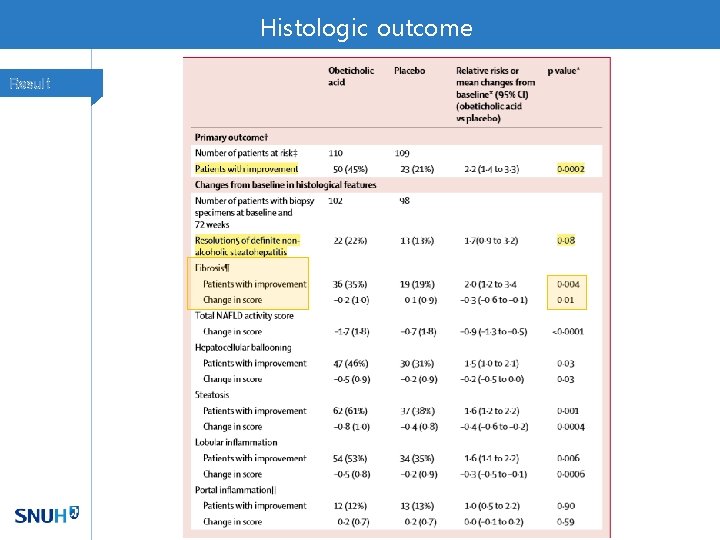
Histologic outcome Result

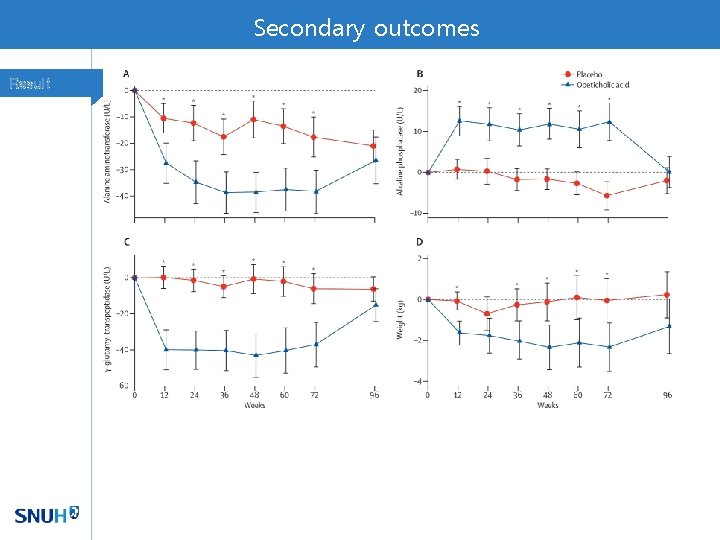
Secondary outcomes Result

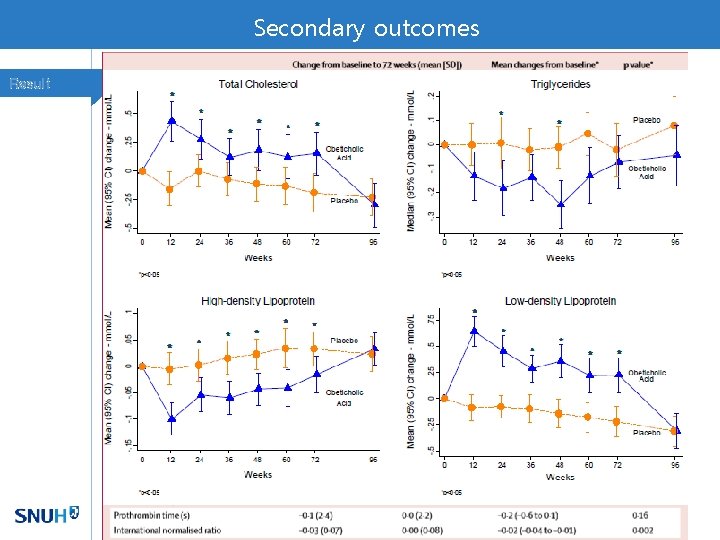
Secondary outcomes Result

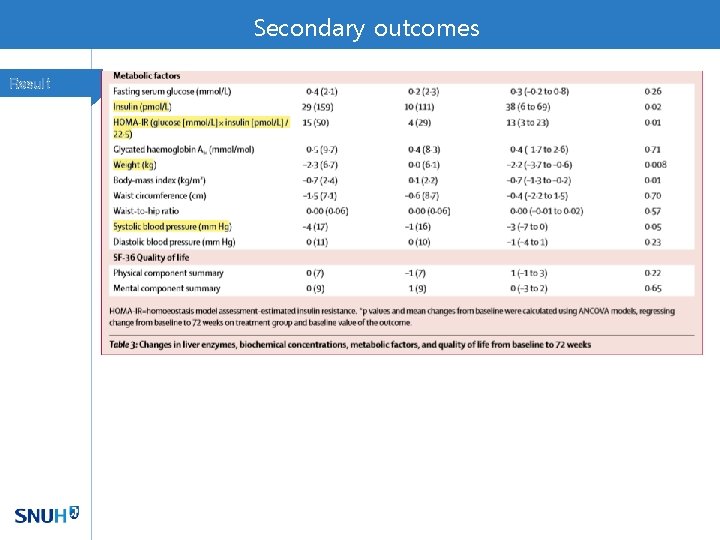
Secondary outcomes Result

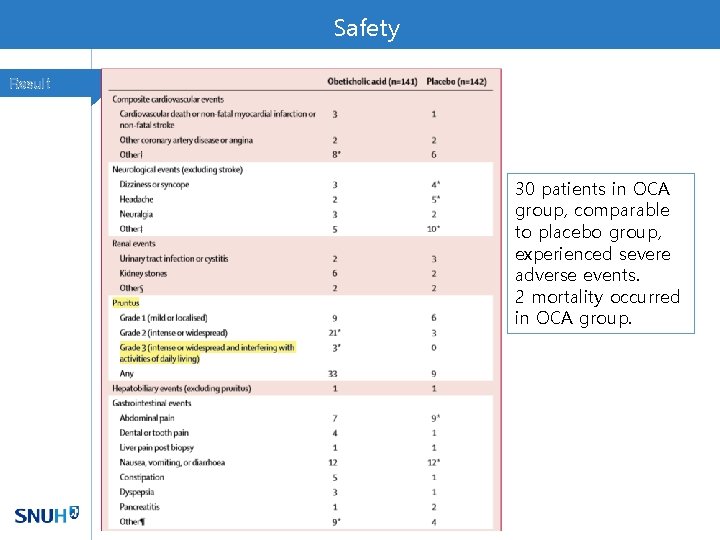
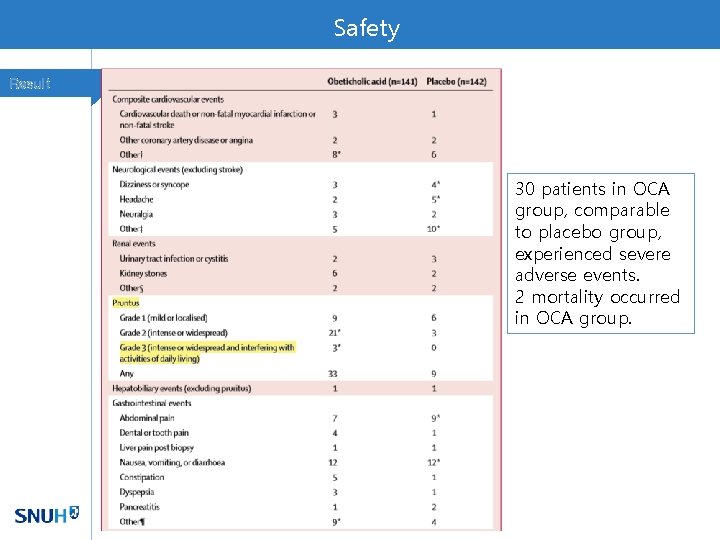
Safety Result 30 patients in OCA group, comparable to placebo group, experienced severe adverse events. 2 mortality occurred in OCA group.


Secondary Analysis of FLINT Trial Method 50/102 in OCA group and 23/98 placebo group showed response

Secondary Analysis of FLINT Trial Method • Objectives: Identify clinical predictors of histologic response • 200 patients (OCA 25 mg n=102; placebo n=98) included in the analysis • Multivariable logistic regression model with stepwise selection • Akaike information criterion (AIC) and Bayesian information criterion (BIC) • Apply penalty on the number of selected predictors


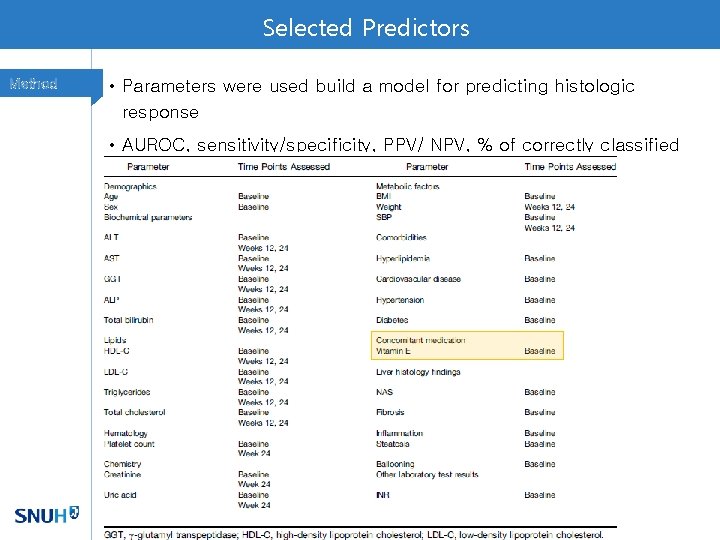
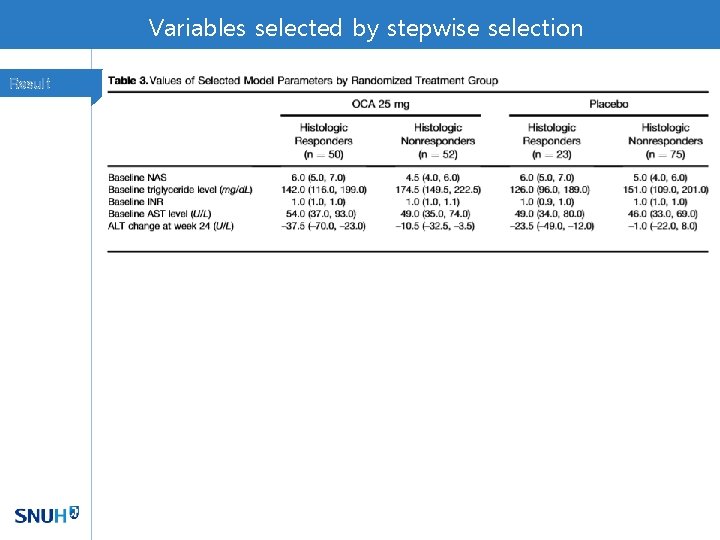
Selected Predictors Method • Parameters were used build a model for predicting histologic response • AUROC, sensitivity/specificity, PPV/ NPV, % of correctly classified cases

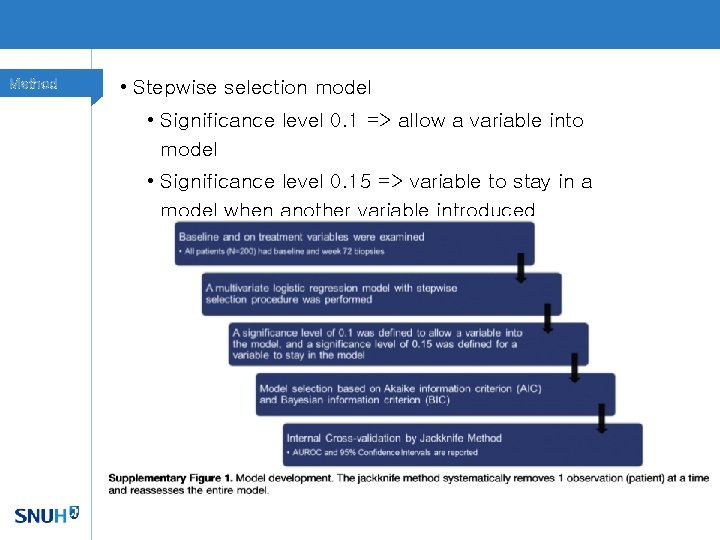
Method • Stepwise selection model • Significance level 0. 1 => allow a variable into model • Significance level 0. 15 => variable to stay in a model when another variable introduced

Baseline characteristics was balanced Result

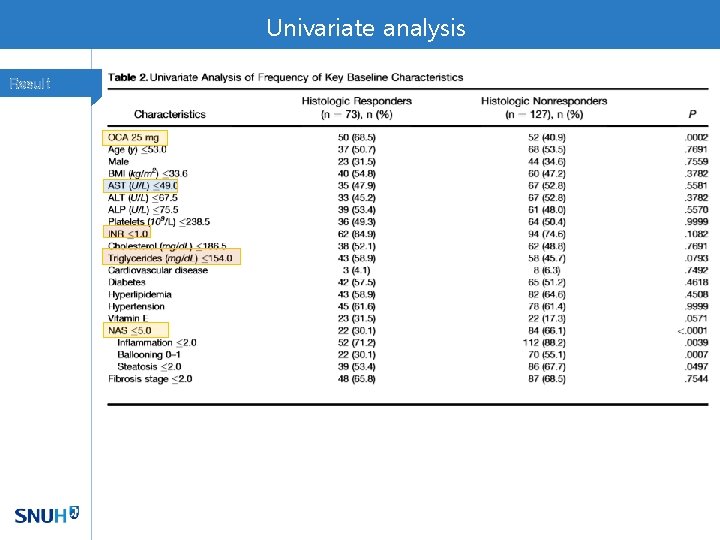
Univariate analysis Result

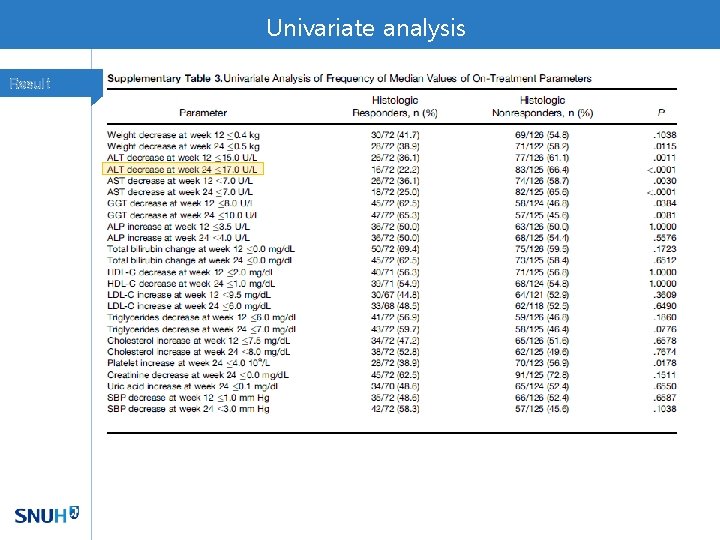
Univariate analysis Result

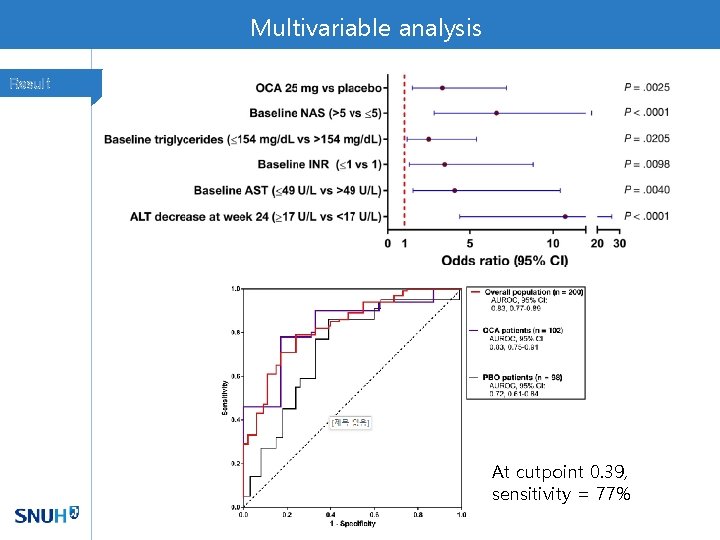
Variables selected by stepwise selection Result

Multivariable analysis Result At cutpoint 0. 39, sensitivity = 77%


Discussion • OCA improved biochemical and histological features of NASH. Fibrosis as well as necroinflammatory change improved. • Obtain liver biopsy specimen before and after the treatment and assessed by expert liver pathologists • Due to multicenter nature, lack of detailed tracking of intervention including dose of statin during trial.

Discussion • Although OCA had an effect on the primary endpoint, did not cause a significant portion to reach resolution of NASH. • NAS score does not fully reflect the status of NASH. Long-term outcome studies are needed to determine the histological measures with greatest clinical significance. • Overall histological improvement rates were similar with PIVENS study • OCA 50/110 (45%), Vitamin E 36/84 (43%), Pioglitazone 27/80 (34%)

Dyslipidemia in OCA group Discussion • FXR activation decreases hepatic lipogenesis by down-regulating SREBP 1 c and increasing SIRT 1 • However, it reduces bile acid synthesis by inhibiting the conversion of cholesterol to bile acids • FXR agonists promote reverse cholesterol transport out of tissue via HDL • Treatment with statin might contributed to the changes toward baseline, should prospectively monitor lipid profile. • Besides 3 patients with severe pruritis, OCA was well tolerable to most of patients

Hepatic insulin resistance Discussion • Previous phase IIa study with OCA showed increased insulin sensitivity via hyperinsulinemic euglycemic clamp technique - Sunder M. et al. Gastroenterology 2013; 145: 574 -5 • Effect of OCA on insulin resistance might be transient and would be reversed by adaptive mechanisms to longterm treatment. • However, along with elevated serum cholesterol pool, insulin resistance could signal an increased risk of atherogenesis.

Factors associated with histologic response in NASH pts Discussion • High NAS score, low baseline TG, INR<1, low baseline AST, early decrease in ALT level are significant predictors • High NAS – quantitatively measurable change more likely • Fasting TG level • Reflection of hepatic secretion and peripheral lipolysis • Lower level of TG reflects higher capacity of liver function improvement • AST, ALT levels are metrics of liver injury • Patients with higher disease activity but still with conserved liver function are likely having potential to improve

Critical Appraisal Discussion • Obethicholic acid regulates lipid metabolism via FXR pathway and coupled with TGR 5, also mediates anti-inflammatory and antifibrotic properties. • OCA groups shows higher insulin resistance at baseline. Along with administration of vitamin E, pioglitazone, statin Tx, this could exaggerates potency of OCA. • Two mortalities (CHF, cardiac infarction) occurred in OCA group and 30/141 patients experienced severe adverse events. Close monitoring with side effect profile would be required in further study, especially cardiac events. • The model predicted low baseline AST to be predictive value for histologic response. There remains further study regarding patients with high necroinflammatory activity.

Baseline Characteristics Result Of 282 patients, Stage 3 fibrosis 63 (22%) and cirrhosis 2 patients

Safety Result 30 patients in OCA group, comparable to placebo group, experienced severe adverse events. 2 mortality occurred in OCA group.

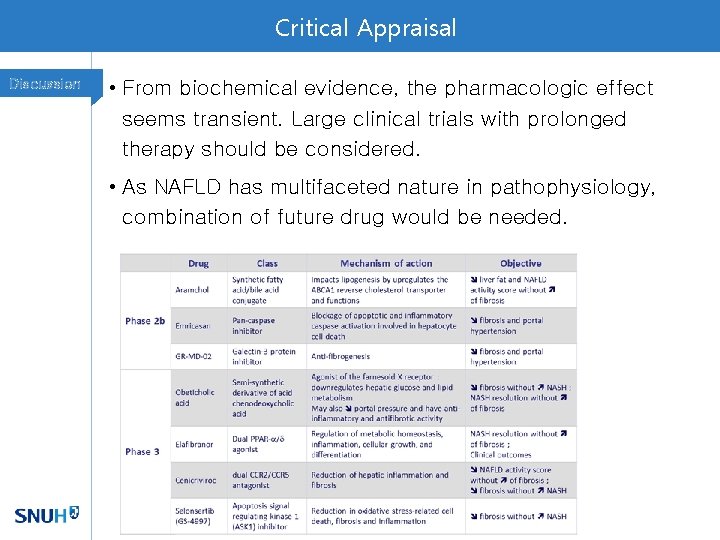
Critical Appraisal Discussion • From biochemical evidence, the pharmacologic effect seems transient. Large clinical trials with prolonged therapy should be considered. • As NAFLD has multifaceted nature in pathophysiology, combination of future drug would be needed.

 Adrenergic agonist
Adrenergic agonist Half life fluoxetine
Half life fluoxetine Cholinergic drugs classification
Cholinergic drugs classification Direct-acting cholinergic drugs
Direct-acting cholinergic drugs Phenylephrine mechanism of action
Phenylephrine mechanism of action Protagonist antagonist and agonist
Protagonist antagonist and agonist Alpha-2 adrenergic
Alpha-2 adrenergic Prime mover of arm extension
Prime mover of arm extension Pectoralis major abduction or adduction
Pectoralis major abduction or adduction Benzodiazepine
Benzodiazepine Glp-1 agonist
Glp-1 agonist Alpha 2 agonist
Alpha 2 agonist Inverse agonist examples
Inverse agonist examples Shoulder flexion agonist and antagonist
Shoulder flexion agonist and antagonist Learning targets
Learning targets Agonist
Agonist B2 agonist inhaler
B2 agonist inhaler Beta agonist hyperglycemia
Beta agonist hyperglycemia Serratus anterior agonist
Serratus anterior agonist Muscarinic agonist
Muscarinic agonist Pectoralis major agonist
Pectoralis major agonist Non catecholamine function
Non catecholamine function Orphan gpcr
Orphan gpcr Nash's pyramid
Nash's pyramid Esercizi equilibrio di nash
Esercizi equilibrio di nash Nash popovic
Nash popovic Equilibrio de nash
Equilibrio de nash Ogletree, deakins, nash, smoak
Ogletree, deakins, nash, smoak John nash hkust
John nash hkust Ogden nash cat poem
Ogden nash cat poem Brewer-nash model (chinese wall)
Brewer-nash model (chinese wall) Pure-strategy nash equilibria
Pure-strategy nash equilibria Nash equilibrium
Nash equilibrium Fossils by ogden nash
Fossils by ogden nash Cystic hygroma
Cystic hygroma Dominant strategy
Dominant strategy Nash evenwicht
Nash evenwicht Nash delirium
Nash delirium Assamad nash
Assamad nash Ogden nash isabel
Ogden nash isabel Bertrand competition
Bertrand competition Neurona motora
Neurona motora