Food Safety Systems Management 1 Training content l





















































- Slides: 65

Food Safety Systems Management 1

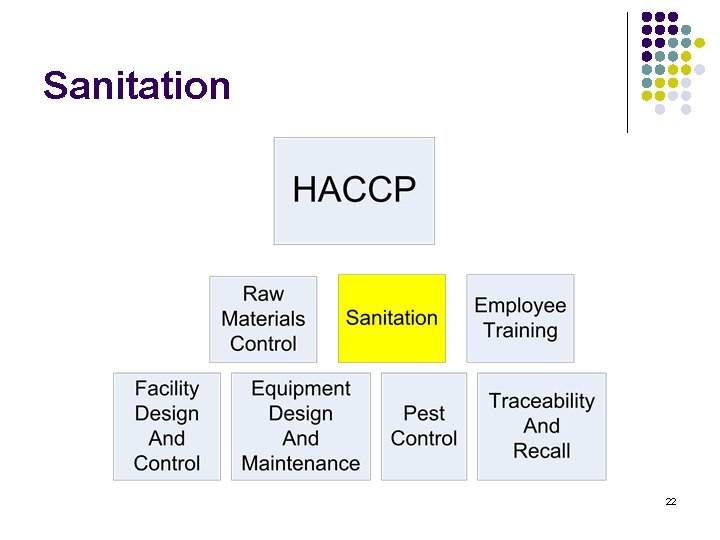
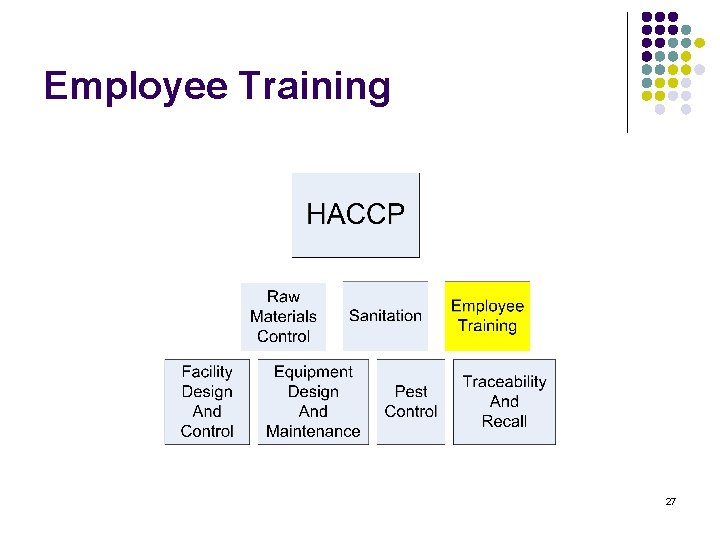
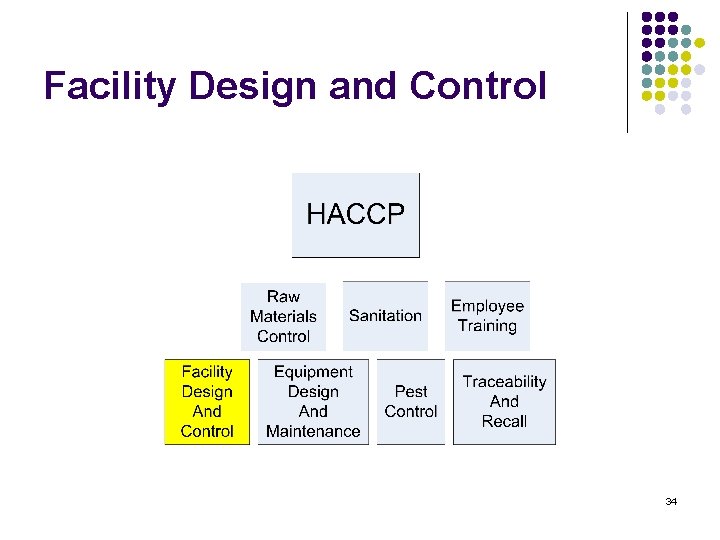
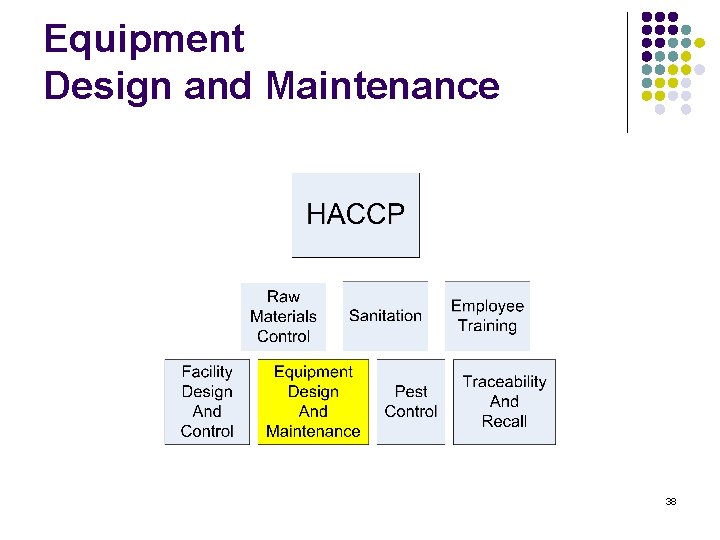
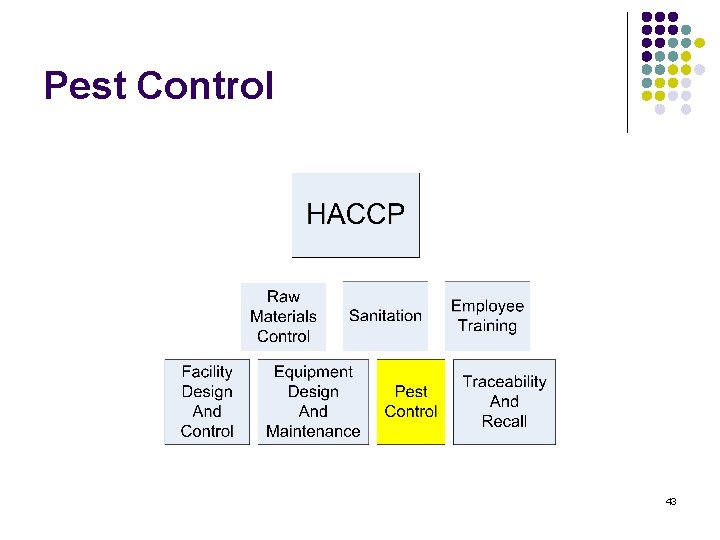
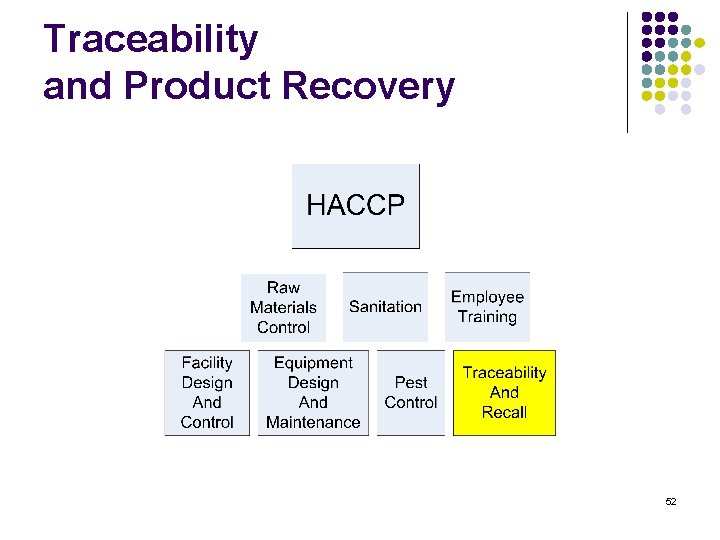
Training content l l l l HACCP Raw materials Control Sanitation Employee training Facility Design and control Equipment Design and maintenance Pest control Traceability and recall 2

Hazard Analysis Critical Control Point- HACCP l l A systematic approach to the identification , evaluation, and control of significant food safety hazards. Risk Assessment and Management tool not unlike a FMEA (Failure Modes Effects Analysis). 3

HA - Hazard Analysis l Hazard: The potential to cause harm. l All food ingredients, food and processes are analyzed for three types of hazards: l l Biological Chemical Physical Hazards may be l l 4 a cause or a factor in food safety

Sources of Hazards 5 l Workers l Materials l Equipment l Processes l Building

Hazards from Source l Workers l Biological § § § l Chemical § l soaps, grease, chemicals Physical § 6 skin, nail, hair colds, sores clothing jewellery, clothing, pens

Hazards from Source l Equipment l Biological § § l Chemical § § § l lubricants cleaning material exhaust Physical § § 7 poor sanitation procedures airflow metal filings parts

Hazards from Source l Building l Biological • • l Chemical § § l storage of chemicals ventilation Physical § 8 walls, floors, openings, storage condensation, water pests employee flow glass, metal, wood

CCP Critical Control Point l CCP: A point, step or procedure at which control can be applied and a food safety hazard can be prevented, eliminated, or reduced to acceptable levels. l Examples of a CCP l l l freezer fryer metal detector label Critical Limit: A value which separates acceptability from unacceptability 9

Biological Hazards How do they become a problem in food ? l Contamination l l l Uncontrolled conditions l inadequate cleaning inappropriate facilities uncontrolled temperatures overcrowding of storage l product exposed to moisture l l from other foods people - hygiene pests equipment & utensils Inadequate destruction l l 10 ineffective sanitation, programs and training inadequate heat treatment

Controlling Biological Hazards l Temperature control l l 11 Keep out of the Danger Zone 40 C - 60 0 C. Atmosphere control Nutrient control Moisture control Prerequisite programs (GMP)

Chemical Hazards l l 12 Cleaners & sanitisers residue Chemicals - lubricants & paint

Chemical Hazards l How do they become a problem in food ? • Contamination l l l • Uncontrolled conditions l l 13 from other foods residues equipment & utensils inappropriate or lack of SOPs inappropriate storage facilities lack of specifications and QC overcrowding of storage

Controlling Chemical Hazards l Appropriate SOPs. l Appropriate prerequisite programs. 14

Physical Hazards l Injurious Extraneous Matter (IEM) l l Examples of IEM - 15 Risk is based on hardness, sharpness, size or shape. metal glass stones jewellery wood plastic

Physical Hazards l How do they become a problem in food ? l Contamination l l l Uncontrolled conditions l l l 16 misuse of packaging broken glass pallets physically damaged product line breakdowns inappropriate or lack of SOPs inappropriate storage facilities lack of specifications and QC

Controlling Physical Hazards l Magnets l Metal detectors l X-ray l Appropriate SOPs. l Appropriate prerequisite programs. 17

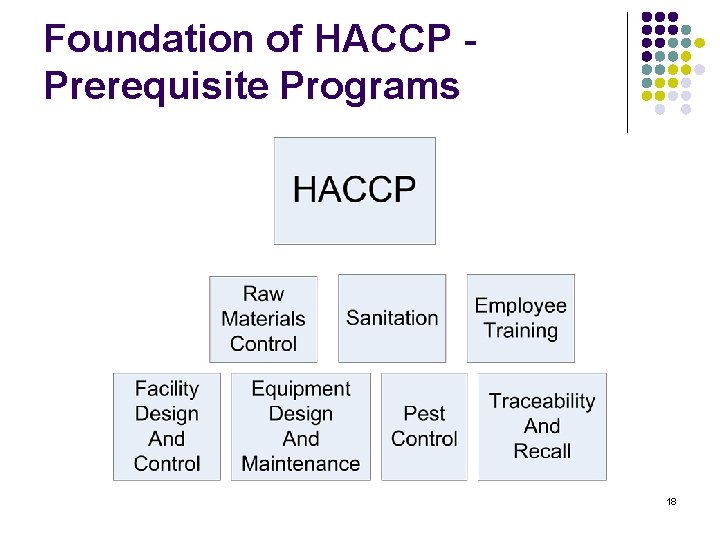
Foundation of HACCP Prerequisite Programs 18

Prerequisite Programs § § § Universal programs / procedures for controlling operational conditions of the plant Create an environment suitable for the production of safe products. Must be adequate and effective. Must be monitored (requires documentation and records) – Internal Audit. Repeated failure of a prerequisite program may indicate that decisions made in a Hazard Analysis are not supported. 19

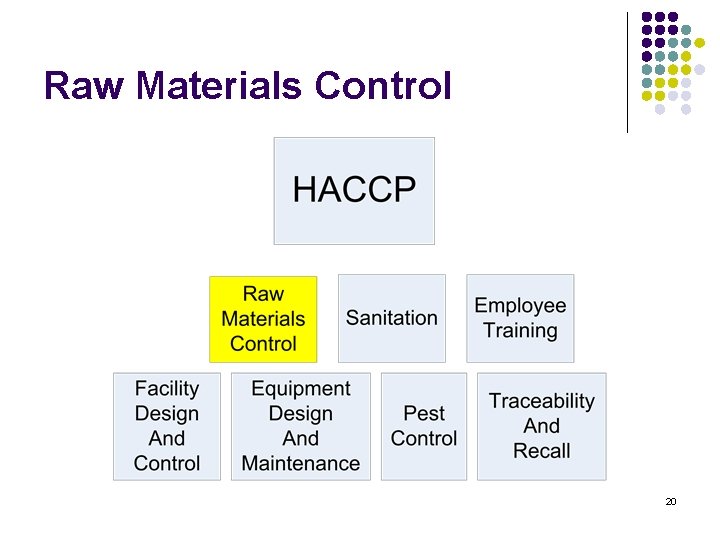
Raw Materials Control 20

Raw Materials Control l Documented inspection of sealed vehicle (temperature, sanitation) in which materials are received. Materials inspected upon receipt. Visual inspection, grading, COA receipt, etc. as your program has defined. Suitably stored to protect quality and integrity of goods. (allergen segregation, temperature, protected, chemicals stored separately and secured, etc). 21

Sanitation 22

Sanitation Program l l 23 Adequacy Adherence

Sanitation Program Areas that must be included in a cleaning program: l l l food storage areas, equipment and work surfaces. toilet and hand washing facilities. lockers, dressing rooms, and lunchroom facilities. storage of clean and soiled linens, clothing and cleaning cloths. garbage and refuse disposal materials and areas. storage of cleaning compounds (poisonous and toxic materials). floors, walls and ceilings. ventilation. lighting. receiving area. entrance, parking lot. 24

Cleaning and Sanitising l Rinse surfaces with warm water. l Wash and scrub as necessary using an approved cleaner as directed in the SSOP. l Rinse surfaces with warm water. l Sanitise using an approved sanitiser as directed in the SSOP. l Time and temperature are critical in cleaning and sanitation. 25

Sanitation l l l l Effective cleaning procedures for equipment and facility. Documented – SSOP (Sanitation Standard Operating Procedures) Defined frequency Chemicals approved for use in food facilities. Records – concentrations, times, temperatures. Sanitation monitoring – visual inspections, chemical or microbiological testing. Corrective actions for deficiencies. 26

Employee Training 27

Employee Training l l Records of training required. Hygienic Practices l Disease Control l Cleanliness – outer garments, hand-washing, unsecured jewelry and other objects, use of gloves, hair and beard nets, eating, drinking, tobacco use, nail polish, etc. Manufacturing Controls l Traffic flow to prevent cross contamination l Ingredient / product handling to protect product l Control measures (p. H, cook time / temp, moisture, etc) l Scheduling sequential runs to prevent cross contamination (as for allergens) Food Defense 28

Personnel Training l Manufacturing controls l Hygienic Practices l l l 29 Communicable disease Injuries Washing of hands Personal cleanliness and conduct Controlled access

Hygiene l The development and maintenance of conditions or practices conducive to good health. l Generally regarded as personal cleanliness and habits. 30

Handwashing l l l l 31 Before starting work. After using the toilet. After touching your hair, ear, nose, mouth. After sneezing, coughing scratching. After eating smoking. After handling a food and before handling another. Before leaving or returning to your work station. After handling garbage and cleaning up.

How to Wash Your Hands l l Use warm running water Use soap (Sing 2 happy birthday song ) Scrub hands together and under nails for at least 15 -20 seconds Dry hands on a clean cloth or paper towel or use an automatic hand dryer if possible 32

Handwashing l To reduce or eliminate transient flora (Microorganisms) l Hands, nails and exposed areas of arms for 30 seconds using soap and under running water. 33

Facility Design and Control 34

Facility Design and Control l Grounds l l l Eliminate pest harborage areas Properly grade roads, yards, parking lots Building l l Provide sufficient space for placement of equipment and storage of materials Prevent cross contamination 35

Facility Design and Control l l l l Floors, walls and ceilings cleaned and in good repair. Prevent condensate Adequate lighting Adequate ventilation Water Quality (potable, steam, ice, reclaimed water, cooling water, backflow prevention) Employee welfare / sanitary facilities Waste control Environmental monitoring 36

Inspection of the physical plant § Inspection should include § § § Slide 37 Immediate environs of the facility External walls, windows, doors and roof Internal walls Ceiling fixtures Floors Module 3. 4 – The Codex General Principles of Food Hygiene –

Equipment Design and Maintenance 38

Equipment Design and Maintenance l l l Designed and of materials and workmanship as to be adequately cleanable – meets standards (e. g. 3 A Sanitary Standards). Installed with adequate space to allow for proper cleaning and maintenance and to prevent cross contamination. Properly maintained (records). Machine cleaning, Machine sanitize Grease, lubricant ( alternative food grade ) or need to sanitize) 39

Cleaning programmes § Cleaning and disinfection programmes should be documented and monitored § Cleaning programmes should specify § Areas, items of equipment and utensils to be cleaned § Responsibility for particular task § Method and frequency of cleaning § Monitoring arrangements Slide 40 Module 3. 4 – The Codex General Principles of Food Hygiene –

Cleaning programmes § Establish appropriate cleaning procedures for each piece of equipment § Cleaned out of place (COP) § Cleaned in place (CIP) § For equipment, disassembly & re-assembly instructions as required for cleaning and inspection Slide 41 Module 3. 4 – The Codex General Principles of Food Hygiene –

Documenting cleaning programmes Programme for weekly cleaning of pulper Procedures: a) Unscrew supports of disks and blades b) Remove disks c) Rinse disks, blades and chassis with clean water, wash with detergent, rinse with clean water a) Reassemble disks and blades b) Tighten the bolts firmly Supervisor: Mr Peter § Cleaning procedures for the equipment § § Slide 42 Institute appropriate daily cleaning procedures Institute more thorough procedures when operation of equipment will be suspended Module 3. 4 – The Codex General Principles of Food Hygiene –

Pest Control 43

Pest Control Program l l 44 Adequacy Adherence

Pest Control l l Prevention is key (design, maintenance and sanitation) Rodent control Insect control Bird / Bat Control 45

Pest control systems - general § Pests pose a major threat to the safety and suitability of food § Pests infestation can occur where there are breeding sites and supply of food § Good hygiene practices should be employed to avoid creating an conducive environment § Good sanitation, inspection of incoming materials and good monitoring can minimise risks of infestation Slide 46 Module 3. 4 – The Codex General Principles of Food Hygiene –

Pest control systems Preventing access § Buildings should be kept in good repair and condition to prevent pest access and to eliminate potential breeding sites § Use of wire mesh screens on windows, doors, ventilators § Holes and drains kept sealed Harbourage and infestation § Availability of food and water encourages pest harbourage and infestation: § Potential food sources should be stored in pest-proof containers or § Stacked above the ground away from walls Slide 47 Module 3. 4 – The Codex General Principles of Food Hygiene –

Pest control systems Monitoring and detection § Establishments and surrounding areas should be regularly examined for evidence of infestation Eradication § Pest infestations should be dealt with immediately and without adversely affecting food safety or suitability § § § Slide 48 Treatments with chemical, physical or biological agents should be carried out without posing a threat to the safety of food Pesticides used should be acceptable to the food control regulatory authorities Where applicable, name of the pest control company or any person contracted for the pest control programme Module 3. 4 – The Codex General Principles of Food Hygiene –

Pest Control l Eliminate entry ways. l Eliminate habitats and food supplies. l Destroy or catch those that gain entry. l Regular inspections l Sound well documented pest control program 49

Pest Control Remember l l l 1 fly leads to 900 1 cockroach leads to 30 - 40 1 moth leads to 400 1 beetle leads to 375 1 rodent leads to 30 Pesticides and rodentcides can only be applied by certified technicians. 50

Waste management § Waste stores and containers must be kept clean § Suitable provision must be taken for the removal, storage and handling of waste § Waste must not be allowed to accumulate in food handling, food storage, and other working areas and adjoining environment Slide 51 Module 3. 4 – The Codex General Principles of Food Hygiene –

Traceability and Product Recovery 52

Traceability and Product Recovery l l Documented program with written procedures to meet federal requirements. Program should include l l l Product Identification / Lot coding Finished product distribution records retained beyond shelf life of product Responsible individuals and their roles Identification of Key Contacts – Internal, supplier customer Mock Recovery Program One step forward, one step back 53

Prerequisite programs are in place Let’s talk HACCP 54

Steps to HACCP Implementation l l l Assemble the HACCP Team Describe the food and its distribution Describe the intended use and consumers of the food Develop a flow diagram which describes the process Verify the Flow Diagram 55

Multidisciplinary HACCP Team l l l Quality Assurance Sanitation Engineering Microbiology Production Outside experts (if necessary) 56

Verify the Flow Diagram l l l HACCP Team should perform an on-site review of the operation to verify the accuracy and completeness of the flow diagram. Take the diagram out to the production floor and walk through the steps. Review periodically, modify and update as necessary (annual review or when change to process occurs). 57

1 - Conduct a Hazard Analysis Identify hazards at each processing step and for each ingredient and material used. l l l Biological – such as pathogen Chemical – such as toxin Physical - such as glass Evaluate hazards to determine severity and likelihood to occur The hazard evaluation provides a basis for determining control measures such as CCP’s 58

2 - Determine Critical Control Points l l A critical control point (CCP) is defined as a step at which control can be applied and is essential to prevent or eliminate a food safety hazard or reduce it to an acceptable level. Information from the hazard analysis should enable the HACCP team to identify which steps in the process are CCPs. 59

3 – Establish Critical Limits l l A critical limit must be scientifically based and is a maximum and / or minimum value to which a biological, chemical or physical parameter must be controlled at a CCP to prevent, eliminate or reduce the food safety hazard to an acceptable level. Examples: Temperature, time, Water Activity, p. H, safe tolerance level for drug residues 60

4 – Establish Monitoring Procedures l l l Monitoring is a planned sequence of observations or measurements used to assess whether a CCP is under control. Monitoring should produce an accurate record for use in verification. Where there is loss of control (a CCP limit is exceeded) there must be documented corrective action. 61

Monitoring l l Continuous is desirable (recording chart) but where not possible, frequency for monitoring must be established. Responsibility for monitoring must be assigned - Position title / work station 62

5 - Establish Corrective Actions l l Corrective actions are procedures to be followed when a deviation occurs. Corrective actions must be specific. l l l Halt production of product Isolate the affected product Return the process to control Determine the disposition of the product Records must be kept for a reasonable period after the shelf life of the product 63

6 _ Establish Verification Procedures l l Establish procedures to verify that the system is working properly. Might include calibration and testing of monitoring equipment, demonstration of system performance, documented record review 64

7 - Records l Identify records that are being used to monitor control points l l l Records of training Records at process step (recording charts, temperature records, etc) Deviation logs Verification and Validation Records to show changes to the HACCP Plan 65